Kupffer-Cell-Targeted Carboxylesterase 1f Knockdown Deteriorates Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure Through Regulating Cellular Polarization in Mice
- PMID: 39734640
- PMCID: PMC11681982
- DOI: 10.1155/cjgh/6410484
Kupffer-Cell-Targeted Carboxylesterase 1f Knockdown Deteriorates Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure Through Regulating Cellular Polarization in Mice
Abstract
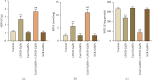
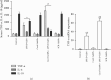
Background: Aims: Carboxylesterase (Ces)1f is implicated in protection against hepatic inflammation, but it is unclear whether the enzyme has an influence in polarization of Kupffer cells (KCs), the innate immune cells mediating hepatic inflammatory injury including acute liver failure (ALF). In the present study, we aim to explore KC polarization induced by Ces1f in mice with lipopolysaccharide/D-galactosamine (LPS/D-GalN)-induced ALF. Methods: We adopted a novel delivery system, β-1,3-D-glucan-encapsulated Endoporter-siRNA particles, to specifically target KC Ces1f knockdown via tail vein injection in mice. Results: Ces1f knockdown increased LPS/D-GalN-induced lethality as well as serum levels of alanine and aspartate transaminases, deteriorated hepatic inflammatory injury, and imbalanced hepatic oxidative stress molecules including myeloperoxidase, malondialdehyde, and superoxide dismutase in ALF. Ces1f knockdown also increased the levels of proinflammatory cytokines (tumor necrosis factor-α and interleukin-6) and decreased the levels of anti-inflammatory cytokine (interleukin-10) in LPS/D-Gal-induced ALF. Ces1f knockdown promoted KC M1 phenotype and marker expression (including CD86 and interleukin-1β), but inhibited M2 phenotype and marker expression (including CD163, CD206, and Arginase 1). Conclusions: Our results suggest that Ces1f plays a hepatoprotective role through regulating KC polarization, which might contribute to anti-inflammatory and antioxidative effects in LPS/D-Gal-induced ALF mice.
Keywords: Kupffer cells; acute liver failure; carboxylesterase 1f; gene knockdown; polarization phenotype.
Copyright © 2024 Sai Zhao et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Tian L., Li W., Yang L., et al. Cannabinoid Receptor 1 Participates in Liver Inflammation by Promoting M1 Macrophage Polarization via RhoA/NF-Κb P65 and ERK1/2 Pathways, Respectively, in Mouse Liver Fibrogenesis. Frontiers in Immunology . 2017;8:p. 1214. doi: 10.3389/fimmu.2017.01214. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

