Clinical impacts of total parenteral nutrition in hematopoietic stem cell transplantation patients with high nutritional risk
- PMID: 39734672
- PMCID: PMC11671266
- DOI: 10.3389/fnut.2024.1495640
Clinical impacts of total parenteral nutrition in hematopoietic stem cell transplantation patients with high nutritional risk
Abstract
Background: Hematopoietic stem cell transplantation (HSCT) patients often receive consecutive intensive chemotherapy, which can lead to gastrointestinal complications and acute graft-versus-host disease (GVHD), placing patients at high nutritional risk.
Aim: This retrospective study aimed to evaluate the benefits of nutritional support in maintaining nutritional status, reducing weight loss without increasing the incidence of catheter-related bloodstream infections (CRBSI) or liver dysfunction, and improving clinical outcomes in HSCT patients at high nutritional risk.
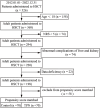
Methods: A total of 526 patients who underwent HSCT were included in the study. Based on the Nutrition Risk Screening-2002 (NRS-2002) and propensity score matching, 70 patients were assigned to the control group (without parenteral nutrition) and 70 to the enhanced nutrition group (with parenteral nutrition) between 2012 and 2022. We compared data between the two groups at different time points (days 3, 7, 10, and 14 after transplantation and the day before discharge) on the following: (1) effectiveness: weight loss, albumin, and prealbumin levels; (2) safety: incidence of CRBSI and conjugated bilirubin levels; and (3) clinical outcomes: hospital stay duration, rate of rehospitalization, hospitalization costs, and survival rates.
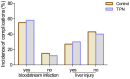
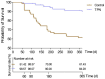
Results: Our results showed that total parenteral nutrition (TPN) effectively mitigated weight loss on days 10 and 14 and the day before discharge, while also improving albumin (33.41 ± 4.57 in the control group, 34.87 ± 4.08 in the TPN group, p < 0.05; 33.72 ± 3.52 in the control group, 35.27 ± 4.04 in the TPN group, p < 0.05; 34.09 ± 4.44 in the control group, 35.55 ± 3.87 in the TPN group, p < 0.05) and prealbumin (245.18 ± 79.94 in the control group, 274.26 ± 86.73 in the TPN group, p < 0.05; 233.27 ± 79.57 in the control group, 279.34 ± 80.20 in the TPN group, p < 0.01; 247.24 ± 83.29 in the control group, 280.65 ± 100.22 in the TPN group, p < 0.05) levels during the same periods. In addition, there were no significant differences in CRBSI incidence or liver function between the non-TPN and TPN groups. Furthermore, the TPN group experienced a shorter length of hospital stay (48.06 ± 13.90 in the control group, 42.13 ± 14.22* in the TPN group, p < 0.05) and lower rates of unexpected rehospitalization (37.1% in the control group, 21.4% in the TPN group, p < 0.05).
Conclusion: This study demonstrated that effective TPN formulations improved nutritional status, ensured patient safety, and contributed to better clinical outcomes in HSCT patients at high nutritional risk. These findings support the use of nutritional interventions in hematologic malignancy patients receiving induction therapy prior to transplantation.
Keywords: catheter-related bloodstream infection; hematopoietic stem cell transplantation; high nutritional risk; liver function; total parenteral nutrition.
Copyright © 2024 Yang, Wu, Dai, Lv and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
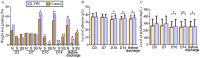

References
-
- August DA, Huhmann MB, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors . A.S.P.E.N. Clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. (2009) 33:472–500. doi: 10.1177/0148607109341804, PMID: - DOI - PubMed
-
- Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. (2024) 11:e147–59. doi: 10.1016/S2352-3026(23)00342-3, PMID: - DOI - PubMed
-
- Fuji S, Kim SW, Kamiya S, Nakane T, Matsumoto K, Onishi Y, et al. A multi-center prospective study randomizing the use of fat emulsion in intensive glucose control after allogeneic hematopoietic stem cell transplantation using a myeloablative conditioning regimen. Clin Nutr. (2018) 37:1534–40. doi: 10.1016/j.clnu.2017.08.022, PMID: - DOI - PubMed
-
- Zama D, Gori D, Muratore E, Leardini D, Rallo F, Turroni S, et al. Enteral versus parenteral nutrition as nutritional support after allogeneic hematopoietic stem cell transplantation: a systematic review and Meta-analysis. Transplant Cell Ther. (2021) 27:180.e181–8. doi: 10.1016/j.jtct.2020.11.006 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

