Growth-promoting effects of self-selected microbial community on wheat seedlings in saline-alkali soil environments
- PMID: 39734744
- PMCID: PMC11671506
- DOI: 10.3389/fbioe.2024.1464195
Growth-promoting effects of self-selected microbial community on wheat seedlings in saline-alkali soil environments
Abstract
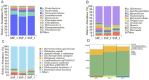
Saline-alkali land is a type of soil environment that causes poor crop growth and low yields. Its management and utilization are, therefore of great significance for increasing arable land resources, ensuring food security, and enhancing agricultural production capacity. The application of plant growth-promoting rhizobacteria (PGPR) is an effective way to promote the establishment of symbiotic relationships between plants and the rhizosphere microenvironment, plant growth and development, and plant resistance to saline-alkali stress. In this study, multiple saline-alkali-resistant bacteria were screened from a saline-alkali land environment and some of them were found to have significantly promotive effects on the growth of wheat seedlings under saline-alkali stress. Using these PGPR, a compound microbial community was selectively obtained from the root-zone soil environment of wheat seedlings, and the metagenomic sequencing analysis of wheat root-zone soil microbiomes was performed. As a result, a compound microbial agent with a Kocuria dechangensis 5-33:Rossellomorea aquimaris S-3:Bacillus subtilis BJYX:Bacillus velezensis G51-1 ratio of 275:63:5:1 was obtained through the self-selection of wheat seedlings. The synthetic compound microbial agent significantly improved the growth of wheat seedlings in saline-alkali soil, as the physiological plant height, aboveground and underground fresh weights, and aboveground and underground dry weights of 21-day-old wheat seedlings were increased by 27.39% (p < 0.01), 147.33% (p < 0.01), 282.98% (p < 0.01), 194.86% (p < 0.01), and 218.60% (p < 0.01), respectively. The promoting effect of this compound microbial agent was also greater than that of each strain on the growth of wheat seedlings. This microbial agent could also regulate some enzyme activities of wheat seedlings and the saline-alkali soil, thereby, promoting the growth of these seedlings. In this study, we analyze an efficient microbial agent and the theoretical basis for promoting the growth of wheat seedlings under saline-alkali stress, thereby, suggesting an important solution for the management and utilization of saline-alkali land.
Keywords: metagenome; plant growth-promoting rhizobacteria; saline-alkali soil environment; the rhizosphere microenvironment; wheat.
Copyright © 2024 Li, Li, Wang, Ji, Han, Gong, Dong, Wang, Zhu, Du, Liu, Jiang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Aghili F., Jan J., Amir H. K., Majid A., Rainer S., Emmanuel F., et al. (2014). Wheat plants invest more in mycorrhizae and receive more benefits from them under adverse than favorable soil conditions. Appl. Soil Ecol. 84, 93–111. 10.1016/j.apsoil.2014.06.013 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

