Association between atherosclerosis and the development of multi-organ pathologies
- PMID: 39734765
- PMCID: PMC11672402
- DOI: 10.1177/20503121241310013
Association between atherosclerosis and the development of multi-organ pathologies
Abstract
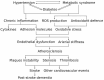
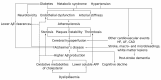
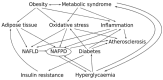
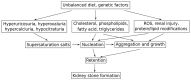
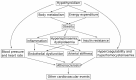
Atherosclerosis is a chronic inflammatory disease affecting the vascular system, characterised by the accumulation of modified lipoproteins, immune cell aggregation and the development of fibrous tissue within blood vessel walls. As atherosclerosis impacts blood vessels, its adverse effects may manifest across various tissues and organs. In this review, we examine the association of atherosclerosis with Alzheimer's disease, stroke, pancreatic and thyroid dysfunction, kidney stones and chronic kidney diseases. In several cases, the reciprocal causative effect of these diseases on the progression of atherosclerosis is also discussed. Particular attention is given to common risk factors, biomarkers and identified molecular mechanisms linking the pathophysiology of atherosclerosis to the dysfunction of multiple tissues and organs. Understanding the role of atherosclerosis and its associated microenvironmental conditions in the pathology of multi-organ disorders may unveil novel therapeutic avenues for the prevention and treatment of cardiovascular and associated diseases.
Keywords: Alzheimer’s disease; Atherosclerosis; chronic kidney disease; kidney stones; stroke; thyroid dysfunction.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

