Diversity and functional traits based indigenous rhizosphere associated phosphate solubilizing bacteria for sustainable production of rice
- PMID: 39735185
- PMCID: PMC11671494
- DOI: 10.3389/fmicb.2024.1470019
Diversity and functional traits based indigenous rhizosphere associated phosphate solubilizing bacteria for sustainable production of rice
Abstract
Introduction: Rice, particularly Basmati rice, holds significant global importance as a staple food. The indiscriminate use of phosphate-based fertilizers during rice production has led to high residual levels of these chemicals in soil, impacting soil health and fertility. This study aimed to address this challenge by investigating the potential of phosphate solubilizing bacteria (PSB) in improving soil fertility and boosting the growth of Basmati rice.
Methods: Using amplicon-based 16S rDNA sequencing, bacterial isolation and cultivation, conducting greenhouse and field experiments, and PSB localization, we optimized the search for PSB inoculants to enhance Basmati rice growth.
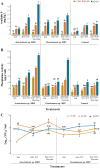
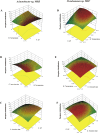
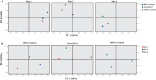
Results and discussion: Rice rhizosphere prokaryote communities showed significant differences in microbial diversity and composition between between basmati and non-basmati rice cultivated areas. Dominant bacterial phyla included Proteobacteria, Acidobacteria, Actinobacteria, and Firmicutes, with Actinobacteria and Proteobacteria playing a crucial role in nutrient recycling. Isolation and optimization of PSB strains, including Acinetobacter sp. MR5 and Pseudomonas sp. R7, were carried out and soil microcosm studies confirmed their efficacy in increasing soil available phosphorus concentration. Response surface methodology revealed the relative importance of factors such as pH, inoculum density and incubation temperature in maximising phosphate solubilization. Microplot experiments demonstrated the effectiveness of optimized PSB inoculants in promoting Basmati rice growth, with significant increases in plant height, tiller number, biomass, and grain yield compared to uninoculated controls. A consortium of PSB proved superior to single-strain inoculants, even with reduced chemical fertilizer application. Field trials at several rice growing sites confirmed the positive impact of the PSB consortium on grain yield, soil phosphorus availability, and plant phosphorus uptake. The competence and persistence of the inoculated strains in the rhizosphere was confirmed by FISH and BOX Polymerase Chain Reaction (BOX-PCR). This work highlights the potential of PSB-based biofertilizers to improve soil fertility, promote sustainable rice production and reduce the negative environmental impacts of chemical fertilizers. Future research would focus on scaling up these findings for widespread adoption in agriculture and exploring their applicability to other crops and agroecosystems.
Keywords: Basmati rice; biofertilizers; microbial diversity; next-generation sequencing; nutrient recycling; sustainable agriculture.
Copyright © 2024 Rasul, Yahya, Suleman, Hakim, Mirza, Mirza, Reitz, Tarkka and Yasmin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Aiba C., Correa R. C., Gottlieb O. R. (1973). Natural occurrence of Erdtman's dehydrodiisoeugenol. Phytochemistry 12, 1163–1164. doi: 10.1016/0031-9422(73)85034-4 - DOI
-
- Albahri G., Alyamani A. A., Badran A., Hijazi A., Nasser M., Maresca M., et al. . (2023). Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 13:1709. doi: 10.3390/agronomy13071709 - DOI
-
- Amardip Singh A. S., Poonam P., Ghosh A. K. (2012). Screening and assessment of phosphate solubilising microbes as potential biofertilizer, isolated from selected wetland and rain-fed ecosystem of Bihar. Asian J. Exp. Biol. Sci. 3, 397–406.
-
- Arai-Sanoh Y., Tsutomu I., Akihiro O., Motohiko K. (2010). Effects of soil temperature on growth and root function in rice. Plant Production Science 13., 235–242.
LinkOut - more resources
Full Text Sources

