Transient Receptor Potential Ankyrin 1 (TRPA1) Mediated LPS-Induced Inflammation in Periodontal Ligament Stem Cells by Inhibiting the Phosphorylation of JNK
- PMID: 39735214
- PMCID: PMC11679270
- DOI: 10.1155/sci/7461604
Transient Receptor Potential Ankyrin 1 (TRPA1) Mediated LPS-Induced Inflammation in Periodontal Ligament Stem Cells by Inhibiting the Phosphorylation of JNK
Abstract
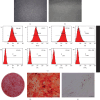
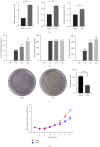
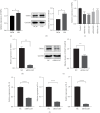
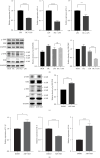
Transient receptor potential ankyrin 1 (TRPA1) molecule is an important type of transient receptor potential (TRP) cation channels, which can cause extracellular Ca2+ to flow into cells after activation. TRPA1 plays an important role in acute and chronic pain, inflammation, kidney disease, cough and asthma, osteoarthritis, cardiovascular disease, obesity, diabetes, and other diseases. In this study, the expression of interleukin (IL)-1β, IL-6, and IL-8 in periodontal ligament stem cells (PDLSCs) treated by lipopolysaccharide (LPS) and the effect of LPS on PDLSCS proliferation were detected. Meanwhile, the change in TRPA1 expression in PDLSCs treated by LPS was also assessed. By knocking down the expression of TRPA1 and using the TRPA1 antagonist HC-030031, the expression of IL-1β, IL-6, and IL-8 in PDLSCs treated by LPS was downregulated. After LPS stimulation, the proliferation ability of PDLSCs decreased, the gene expression and secretion of IL-1β, IL-6, and IL-8 increased and the gene and protein expression of TRPA1 were upregulated. Reducing the expression of TRPA1 can effectively inhibit the increase of gene expression of IL-1β, IL-6, and IL-8 after LPS stimulation, and pretreatment of PDLSCs with HC-030031 can also achieve the above effect. And research has found that HC-030031 can inhibit the phosphorylation level of JNK in PDLSCs treated by LPS. The use of JNK inhibitor JNK-IN-8 can also reduce the expression of IL-1β, IL-6, and IL-8 in PDLSCs. Finally, this study found LPS could cause the upregulation of TRPA1, and the inhibition of TRPA1 could produce an anti-inflammatory effect in PDLSCs treated by LPS due to its inhibition of JNK phosphorylation.
Keywords: JNK; PDLSCs; TRPA1.
Copyright © 2024 Xian Wang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

