The Genetic and Epigenetic Toxicity of Silica Nanoparticles: An Updated Review
- PMID: 39735322
- PMCID: PMC11681786
- DOI: 10.2147/IJN.S486858
The Genetic and Epigenetic Toxicity of Silica Nanoparticles: An Updated Review
Abstract
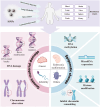
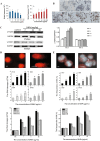
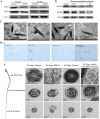
Silica nanoparticles (SiNPs) are widely used in biomedical fields, such as drug delivery, disease diagnosis, and molecular imaging. An increasing number of consumer products containing SiNPs are being used without supervision, and the toxicity of SiNPs to the human body is becoming a major problem. SiNPs contact the human body in various ways and cause damage to the structure and function of genetic material, potentially leading to carcinogenesis, teratogenicity and infertility. This review summarizes SiNPs-induced genetic and epigenetic toxicity, especially to germ cells, and explore their potential mechanisms. SiNPs cause genetic material damage mainly by inducing oxidative stress. Furtherly, the molecular mechanisms of epigenetic toxicity are discussed in detail for the first time. SiNPs alter DNA methylation, miRNA expression, histone modification and inhibit chromatin remodeling by regulating epigenetic-related enzymes and transcription factors. This review is beneficial for investigating potential solutions to avoid toxicity and provide guidance for better application of SiNPs in the biomedical field.
Keywords: DNA damage; epigenetic; genotoxicity; germ cells; silica nanoparticles.
© 2024 Zheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
The toxicity of silica nanoparticles to the immune system.Nanomedicine (Lond). 2018 Aug 1;13(15):1939-1962. doi: 10.2217/nnm-2018-0076. Epub 2018 Aug 28. Nanomedicine (Lond). 2018. PMID: 30152253 Review.
-
Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles.Int J Nanomedicine. 2016 Mar 7;11:919-28. doi: 10.2147/IJN.S92278. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27022259 Free PMC article.
-
Advances in the study of silica nanoparticles in lung diseases.Sci Total Environ. 2024 Feb 20;912:169352. doi: 10.1016/j.scitotenv.2023.169352. Epub 2023 Dec 16. Sci Total Environ. 2024. PMID: 38110102 Review.
-
Silica nanoparticles: Biomedical applications and toxicity.Biomed Pharmacother. 2022 Jul;151:113053. doi: 10.1016/j.biopha.2022.113053. Epub 2022 May 17. Biomed Pharmacother. 2022. PMID: 35594717 Review.
-
Reproductive toxicity investigation of silica nanoparticles in male pubertal mice.Environ Sci Pollut Res Int. 2022 May;29(24):36640-36654. doi: 10.1007/s11356-021-18215-6. Epub 2022 Jan 22. Environ Sci Pollut Res Int. 2022. PMID: 35064498
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

