Injectable Polyhydroxyalkanoate-Nano-Clay Microcarriers Loaded with r-BMSCs Enhance the Repair of Cranial Defects in Rats
- PMID: 39735323
- PMCID: PMC11681809
- DOI: 10.2147/IJN.S498950
Injectable Polyhydroxyalkanoate-Nano-Clay Microcarriers Loaded with r-BMSCs Enhance the Repair of Cranial Defects in Rats
Abstract
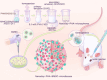
Purpose: Successful regeneration of cranial defects necessitates the use of porous bone fillers to facilitate cell proliferation and nutrient diffusion. Open porous microspheres, characterized by their high specific surface area and osteo-inductive properties, offer an optimal microenvironment for cell ingrowth and efficient ossification, potentially accelerating bone regeneration.
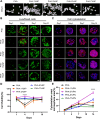
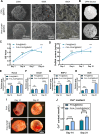
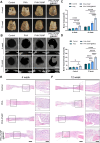
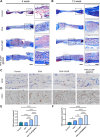
Materials and methods: An in vitro investigation was conducted to assess the physicochemical properties, porosity, and biocompatibility of PHA-nano-clay open porous microspheres. Subsequently, PHA-nano-clay microspheres loaded with rat bone marrow mesenchymal stem cells were implanted into 5 mm cranial defects in rats for a duration of 12 weeks and were evaluated through histological and immunohistochemical analyses.
Results: The incorporation of nano-clay into PHA resulted in improved mechanical properties of the porous scaffolds. Furthermore, cell adhesion, viability, and morphology on the scaffolds were maintained. The PHA-3% nano-clay open porous microspheres effectively enhanced the repair of cranial defects compared to the control group, without recurrence or complications.
Conclusion: Porous PHA-nano-clay microspheres, with their high specific surface area, biodegradability, and osteo-inductive properties, can be utilized as a bone-filling material for improved bone defect repair through cell delivery. In particular, PHA-3% nano-clay open porous microspheres exhibit promising therapeutic potential in the repair of cranial defects.
Keywords: P34HB; bone-filling biomaterial; cranial defects; open porous microspheres; osteo-inductivity.
© 2024 Ci et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

