Recent Advances and Future Directions in Sonodynamic Therapy for Cancer Treatment
- PMID: 39735354
- PMCID: PMC11671681
- DOI: 10.34133/bmef.0080
Recent Advances and Future Directions in Sonodynamic Therapy for Cancer Treatment
Abstract
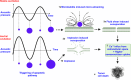
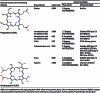
Deep-tissue solid cancer treatment has a poor prognosis, resulting in a very low 5-year patient survival rate. The primary challenges facing solid tumor therapies are accessibility, incomplete surgical removal of tumor tissue, the resistance of the hypoxic and heterogeneous tumor microenvironment to chemotherapy and radiation, and suffering caused by off-target toxicities. Here, sonodynamic therapy (SDT) is an evolving therapeutic approach that uses low-intensity ultrasound to target deep-tissue solid tumors. The ability of ultrasound to deliver energy safely and precisely into small deep-tissue (>10 cm) volumes makes SDT more effective than conventional photodynamic therapy. While SDT is currently in phase 1/2 clinical trials for glioblastoma multiforme, its use for other solid cancer treatments, such as breast, pancreatic, liver, and prostate cancer, is still in the preclinical stage, with further investigation required to improve its therapeutic efficacy. This review, therefore, focuses on recent advances in SDT cancer treatments. We describe the interaction between ultrasound and sonosensitizer molecules and the associated energy transfer mechanism to malignant cells, which plays a central role in SDT-mediated cell death. Different sensitizers used in clinical and preclinical trials of various cancer treatments are listed, and the critical ultrasound parameters for SDT are reviewed. We also discuss approaches to improve the efficacies of these sonosensitizers, the role of the 3-dimensional spheroid in vitro investigations, ultrasound-controlled CAR-T cell and SDT-based multimodal therapy, and machine learning for sonosensitizer optimization, which could facilitate clinical translation of SDT.
Copyright © 2024 Priyankan Datta et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures















Similar articles
-
In Vitro Sonodynamic Therapy Using a High Throughput 3D Glioblastoma Spheroid Model with 5-ALA and TMZ Sonosensitizers.Adv Healthc Mater. 2024 Dec;13(32):e2402877. doi: 10.1002/adhm.202402877. Epub 2024 Oct 21. Adv Healthc Mater. 2024. PMID: 39434433 Free PMC article.
-
Sonodynamic and Acoustically Responsive Nanodrug Delivery System: Cancer Application.Int J Nanomedicine. 2024 Nov 11;19:11767-11788. doi: 10.2147/IJN.S496028. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39553460 Free PMC article. Review.
-
Sonodynamic therapy and magnetic resonance-guided focused ultrasound: new therapeutic strategy in glioblastoma.J Neurooncol. 2023 May;163(1):219-238. doi: 10.1007/s11060-023-04333-3. Epub 2023 May 14. J Neurooncol. 2023. PMID: 37179515 Free PMC article. Review.
-
A review of sonodynamic therapy for brain tumors.Neurosurg Focus. 2024 Sep 1;57(3):E7. doi: 10.3171/2024.6.FOCUS24338. Neurosurg Focus. 2024. PMID: 39217635 Review.
-
Design and Challenges of Sonodynamic Therapy System for Cancer Theranostics: From Equipment to Sensitizers.Adv Sci (Weinh). 2021 Mar 12;8(10):2002178. doi: 10.1002/advs.202002178. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026428 Free PMC article. Review.
Cited by
-
Biomaterials mediated 3R (remove-remodel-repair) strategy: holistic management of Helicobacter pylori infection.J Nanobiotechnology. 2025 Jul 1;23(1):475. doi: 10.1186/s12951-025-03455-2. J Nanobiotechnology. 2025. PMID: 40598208 Free PMC article. Review.
References
-
- Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1(5):453–458. - PubMed
-
- Lei X, Lei Y, Li JK, du WX, Li RG, Yang J, Li J, Li F, Tan HB. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. - PubMed
LinkOut - more resources
Full Text Sources

