Eculizumab in thymoma-associated myasthenia gravis: a real-world cohort study
- PMID: 39735403
- PMCID: PMC11672488
- DOI: 10.1177/17562864241309431
Eculizumab in thymoma-associated myasthenia gravis: a real-world cohort study
Abstract
Background: Thymoma-associated myasthenia gravis (TAMG) is a subtype of myasthenia gravis (MG) that is associated with more severe symptoms and a relatively poor prognosis. Eculizumab, an inhibitor to target human C5 component of the complement cascade, is considered a treatment option for refractory generalized MG (gMG).
Objectives: To explore the safety and efficacy of eculizumab in patients with TAMG.
Design: This is an observational multicenter real-world cohort study to assess TAMG who were treated with eculizumab from June 2023 to June 2024.
Data sources and methods: Clinical features associated with thymoma-associated multi-organ autoimmunity (TAMA), Myasthenia Gravis Activities of Daily Living (MG-ADL) score, and the incidence of treatment-emergent adverse events (TEAEs) were prospectively collected.
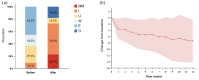
Results: Overall, 42 patients with gMG were treated with eculizumab at 5 research centers, of whom 22 patients with TAMG were finally included. This cohort had a mean age of 51.5 ± 12.1 years and an average disease duration of 4.0 ± 4.3 years. Regarding thymomas, the World Health Organization (WHO) histological classification was primarily B2 and B3 (63.7%), and Masaoka staging was predominantly IV (45.5%). Nine participants (40.9%) switched from efgartigimod to eculizumab aiming at a better clinical improvement and reducing steroid use. By week 12, the MG-ADL score decreased to 4.8 ± 4.7 (baseline: 11.7 ± 6.0), and the corticosteroid dose reduced to 23.2 ± 26.5 mg (baseline: 41.8 ± 63.9 mg). Two patients with TAMA showed significant improvement in skin lesions and thrombocytopenia. Two TEAEs were recorded including COVID-19 and herpes labialis infection. Four patients (18.2%) died of respiratory or circulatory failure owing to thymoma metastasis.
Conclusion: This real-world study demonstrates the efficacy of eculizumab in achieving symptom control and corticosteroid reduction for TAMG. It may also be a therapeutic option for refractory TAMG and TAMA.
Trial registration: NCT04535843.
Keywords: TAMA; TAMG; corticosteroid; eculizumab; refractory.
Plain language summary
Eculizumab in thymoma-associated myasthenia gravis Patients with unresectable thymomas tend to have more severe symptoms at a younger age, a higher frequency of myasthenic crisis (MC), longer respiratory support duration, and increased mortality compared to those without thymomas. Only a few case reports have explored the therapeutic efficacy of eculizumab in thymoma-associated myasthenia gravis (TAMG), which is a subtype of myasthenia gravis (MG) that is associated with anti-acetylcholine receptor antibodies. Using a multicenter, observational cohort of patients with generalized myasthenia gravis from China, we enrolled patients with TAMG who were refractory to immunotherapies and then switched to eculizumab. Our study revealed a significant improvement in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score in patients with TAMG (7.4 points) by week 12. Moreover, this study demonstrated the efficacy and safety of eculizumab in the TAMG cohort, with most patients experiencing clinical improvement within 2 weeks and an average reduction in steroid dosage of approximately one-third in the first month. Further, we believe that this article will be of interest to the readership of your journal because it provides evidence-based findings that indicate that eculizumab has emerged as a viable treatment option not only for TAMG but also for thymoma-associated multiorgan autoimmunity (TAMA).
© The Author(s), 2024.
Figures

Similar articles
-
Patterns and predictors of therapeutic response to efgartigimod in acetylcholine receptor-antibody generalized myasthenia gravis subtypes.Ther Adv Neurol Disord. 2025 Feb 18;18:17562864251319656. doi: 10.1177/17562864251319656. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 39974170 Free PMC article.
-
Perioperative Safety and Efficacy of Efgartigimod for Thymoma-Associated Myasthenia Gravis: A Prospective, Multicenter, Phase II Clinical Trial.J Thorac Oncol. 2025 Aug;20(8):1120-1130. doi: 10.1016/j.jtho.2025.04.014. Epub 2025 May 2. J Thorac Oncol. 2025. PMID: 40320172 Clinical Trial.
-
Complement inhibitor therapy in thymoma-associated myasthenia gravis: a real-world experience.Front Immunol. 2025 Apr 14;16:1562419. doi: 10.3389/fimmu.2025.1562419. eCollection 2025. Front Immunol. 2025. PMID: 40297583 Free PMC article.
-
Eculizumab treatment for myasthenia gravis subgroups: 2021 update.J Neuroimmunol. 2022 Jan 15;362:577767. doi: 10.1016/j.jneuroim.2021.577767. Epub 2021 Nov 18. J Neuroimmunol. 2022. PMID: 34823117 Review.
-
Efficacy and Safety of Immunotherapies in Refractory Myasthenia Gravis: A Systematic Review and Meta-Analysis.Front Neurol. 2021 Dec 1;12:725700. doi: 10.3389/fneur.2021.725700. eCollection 2021. Front Neurol. 2021. PMID: 34925206 Free PMC article.
Cited by
-
Ravulizumab for generalized Myasthenia Gravis: a multicenter real-life experience.J Neurol. 2025 May 14;272(6):396. doi: 10.1007/s00415-025-13127-8. J Neurol. 2025. PMID: 40366475 Free PMC article.
-
The Role of Complement in the Pathogenesis and Treatment of Myasthenia Gravis.Cells. 2025 May 19;14(10):739. doi: 10.3390/cells14100739. Cells. 2025. PMID: 40422242 Free PMC article. Review.
References
-
- Gilhus NE. Myasthenia gravis. N Engl J Med 2016; 375: 2570–2581. - PubMed
-
- Murai H, Utsugisawa K, Motomura M, et al.. The Japanese clinical guidelines 2022 for myasthenia gravis and Lambert–Eaton myasthenic syndrome. Clin Exp Neuroimmunol 2023; 14: 19–27.
-
- Álvarez-Velasco R, Gutiérrez-Gutiérrez G, Trujillo JC, et al.. Clinical characteristics and outcomes of thymoma-associated myasthenia gravis. Eur J Neurol 2021; 28: 2083–2091. - PubMed
-
- Kharnas SS, Ippolitov LI, Fat’ianova AS. [Long-term prognostic analysis of surgical treatment of the thymoma-associated myasthenia gravis]. Khirurgiia (Mosk) 2009; (7): 47–54. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

