Risk assessment and triage strategy of cervical cancer primary screening on HPV integration status: 5-year follow-up of a prospective cohort study
- PMID: 39735440
- PMCID: PMC11674434
- DOI: 10.1016/j.jncc.2024.08.001
Risk assessment and triage strategy of cervical cancer primary screening on HPV integration status: 5-year follow-up of a prospective cohort study
Abstract
Objective: We investigated the relation between man papillomavirus (HPV) integration status and the immediate risk of cervical intraepithelial neoplasia (CIN), as well as the triage strategy based on HPV integration test.
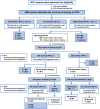
Methods: 4086 women aged 20 to 65 years in China were enrolled in 2015 for a prospective, population-based, clinical observational study to evaluate the triage performance of HPV integration. Cervical exfoliated cells were collected for HPV testing and cytologic test. If high-risk HPV was positive, HPV integration test was performed at baseline, 2-year and 5-year follow-up.
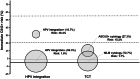
Results: At baseline, HPV integration was positively correlated with the severity of cervical pathology, ranging from 5.0% (15/301) in normal diagnosis, 6.9% (4/58) in CIN1, 31.0% (9/29) in CIN2, 70% (14/20) in CIN3, and 100% (2/2) in cervical cancer (P < 0.001). Compared with cytology, HPV integration exhibits comparable sensitivity and negative predictive value for the diagnosis of CIN3+, higher specificity (92.8% [90.2%-95.4%] vs. 75.5% [71.2%-79.8%], P < 0.001) and higher positive predictive value (36.4% [22.1%-50.6%] vs. 15.2% [8.5%-21.8%], P < 0.001). HPV integration testing strategy yielded a significantly lower colposcopy referral rate than cytology strategy (10.7% [44/410] vs. 27.3% [112/410], P < 0.001). The HPV integration-negative group exhibited the lowest immediate risk for CIN3+ (1.6%) and accounted for the largest proportion of the total population (89.3%), when compared with the normal cytology group (risk, 1.7%; proportion, 72.7%).
Conclusion: As a key molecular basis for the development of cervical cancer, HPV integration might be a promising triage strategy for HPV-positive patients.
Keywords: Cervical cancer screening; Cervical intraepithelial neoplasia; Colposcopy; HPV integration; Human papillomavirus.
© 2024 Chinese National Cancer Center. Published by Elsevier B.V.
Conflict of interest statement
Ding Ma and Zheng Hu are inventors of a patent (ZL201510217845.0) for the HPV integration test. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- WHO guideline for screening and treatment of cervical precancer lesions for cervical cancer prevention (Second edition): WHO Guidelines. 2021 https://www.who.int/publications/i/item/9789240030824 Accessed April 20, 2024.
-
- Molina MA, Steenbergen RDM, Pumpe A, et al. HPV integration and cervical cancer: a failed evolutionary viral trait. Trends Mol Med. 2024;30(9):890–902. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

