Disseminated tumor cells in bone marrow as predictive classifiers for small cell lung cancer patients
- PMID: 39735446
- PMCID: PMC11674436
- DOI: 10.1016/j.jncc.2024.07.003
Disseminated tumor cells in bone marrow as predictive classifiers for small cell lung cancer patients
Abstract
Background: Small cell lung cancer (SCLC) is a highly aggressive disease characterized by early metastasis. Aneuploid CD31- disseminated tumor cells (DTCs) and CD31+ disseminated tumor endothelial cells (DTECs) residing in the bone marrow are generally considered as the initiators of metastatic process. However, the clinical significance of DTCs and DTECs in SCLC remains poorly understood. The aim of this study is to investigate the clinical implications of diverse subtypes of highly heterogeneous DTCs and DTECs in SCLC patients.
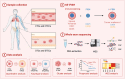
Methods: Subtraction enrichment and immunostaining-fluorescence in situ hybridization (SE-iFISH) was applied to enrich and perform comprehensive morphologic, karyotypic, and phenotypic characterization of aneuploid DTCs and DTECs in 30 patients. Additionally, co-detection of circulating tumor cells (CTCs) and circulating tumor endothelial cells (CTECs) was conducted on 24 of the enrolled patients. Proof-of-concept of the whole exon sequencings (WES) on precisely selected different subtypes of CTCs or DTCs, longitudinally detected from a representative case with pathologically confirmed bone marrow metastasis, was validated to feasibly reveal genetic mutations in these cells.
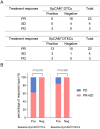
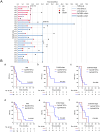
Results: DTCs, DTECs and their subtypes were readily detectable in SCLC patients. Comparative analysis revealed that the number of DTCs and DTECs was significantly higher than that of their corresponding CTCs and CTECs (P < 0.001 for both). Positive detection of disseminated tumor microemboli (DTM) or disseminated tumor endothelial microemboli (DTEM) was associated with inferior survival outcomes (P = 0.046 and P = 0.048). Patients with EpCAM+ DTCs detectable displayed significantly lower disease control rate (DCR) (16.67% vs 73.33%, P = 0.019), reduced median progression-free survival (mPFS) and median overall survival (mOS) compared with those with EpCAM- DTCs (P = 0.028 and P = 0.002, respectively). WES analysis indicated that post-treatment DTCs isolated from bone marrow at the time of disease progression shared more homologous somatic gene mutations with pre-treatment CTCs compared with post-treatment CTCs.
Conclusions: Our findings suggest that bone marrow sampling and characterization of DTC subtypes provided a valuable tool for predicting treatment response and the prognosis in SCLC. Moreover, DTCs inherit a greater amount of homologous somatic information from pre-treatment CTCs, indicating their potential role in disease progression and treatment resistance.
Keywords: Aneuploid DTCs and DTECs; Bone marrow; Prognosis; SCLC; SE-iFISH.
© 2024 Chinese National Cancer Center. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
[Aprospective study of detection and clinical significance of bone marrow tumor cells in small cell lung cancer].Zhonghua Zhong Liu Za Zhi. 2024 May 23;46(5):419-427. doi: 10.3760/cma.j.cn112152-20231014-00193. Zhonghua Zhong Liu Za Zhi. 2024. PMID: 38742355 Chinese.
-
Longitudinal detection of subcategorized CD44v6+ CTCs and circulating tumor endothelial cells (CTECs) enables novel clinical stratification and improves prognostic prediction of small cell lung cancer: A prospective, multi-center study.Cancer Lett. 2023 Sep 1;571:216337. doi: 10.1016/j.canlet.2023.216337. Epub 2023 Aug 6. Cancer Lett. 2023. PMID: 37553013
-
Integrated EpCAM-independent subtraction enrichment and iFISH strategies to detect and classify disseminated and circulating tumors cells.Clin Transl Med. 2015 Dec;4(1):38. doi: 10.1186/s40169-015-0081-2. Epub 2015 Dec 30. Clin Transl Med. 2015. PMID: 26718583 Free PMC article.
-
Biology and significance of circulating and disseminated tumour cells in colorectal cancer.Langenbecks Arch Surg. 2012 Apr;397(4):535-42. doi: 10.1007/s00423-012-0917-9. Epub 2012 Feb 15. Langenbecks Arch Surg. 2012. PMID: 22350614 Review.
-
Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy.J Cell Mol Med. 2018 Dec;22(12):5776-5786. doi: 10.1111/jcmm.13867. Epub 2018 Sep 26. J Cell Mol Med. 2018. PMID: 30255991 Free PMC article. Review.
Cited by
-
In situ phenotypic and karyotypic co-detection of aneuploid TCs and TECs in cytological specimens with abnormal cervical screening results.BMC Cancer. 2025 May 26;25(1):945. doi: 10.1186/s12885-025-14346-y. BMC Cancer. 2025. PMID: 40420028 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

