The impact of Parkinson's disease on striatal network connectivity and corticostriatal drive: An in silico study
- PMID: 39735495
- PMCID: PMC11674317
- DOI: 10.1162/netn_a_00394
The impact of Parkinson's disease on striatal network connectivity and corticostriatal drive: An in silico study
Abstract
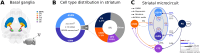
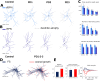
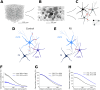
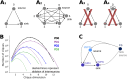
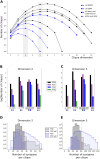
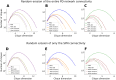
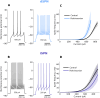
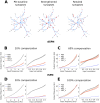
Striatum, the input stage of the basal ganglia, is important for sensory-motor integration, initiation and selection of behavior, as well as reward learning. Striatum receives glutamatergic inputs from mainly cortex and thalamus. In rodents, the striatal projection neurons (SPNs), giving rise to the direct and the indirect pathway (dSPNs and iSPNs, respectively), account for 95% of the neurons, and the remaining 5% are GABAergic and cholinergic interneurons. Interneuron axon terminals as well as local dSPN and iSPN axon collaterals form an intricate striatal network. Following chronic dopamine depletion as in Parkinson's disease (PD), both morphological and electrophysiological striatal neuronal features have been shown to be altered in rodent models. Our goal with this in silico study is twofold: (a) to predict and quantify how the intrastriatal network connectivity structure becomes altered as a consequence of the morphological changes reported at the single-neuron level and (b) to investigate how the effective glutamatergic drive to the SPNs would need to be altered to account for the activity level seen in SPNs during PD. In summary, we predict that the richness of the connectivity motifs in the striatal network is significantly decreased during PD while, at the same time, a substantial enhancement of the effective glutamatergic drive to striatum is present.
Keywords: Computational modeling; Directed cliques; Network higher order connectivity; Neuronal degeneration model; Parkinson’s disease; Striatum; Topological data analysis.
Plain language summary
This in silico study predicts the impact that the single-cell neuronal morphological alterations will have on the striatal microcircuit connectivity. We find that the richness in the topological striatal motifs is significantly reduced in Parkinson’s disease (PD), highlighting that just measuring the pairwise connectivity between neurons gives an incomplete description of network connectivity. Moreover, we predict how the resulting electrophysiological changes of striatal projection neuron excitability together with their reduced number of dendritic branches affect their response to the glutamatergic drive from the cortex and thalamus. We find that the effective glutamatergic drive is likely significantly increased in PD, in accordance with the hyperglutamatergic hypothesis.
© 2024 Massachusetts Institute of Technology.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
References
LinkOut - more resources
Full Text Sources
