ATP5J regulates microglial activation via mitochondrial dysfunction, exacerbating neuroinflammation in intracerebral hemorrhage
- PMID: 39735538
- PMCID: PMC11671693
- DOI: 10.3389/fimmu.2024.1509370
ATP5J regulates microglial activation via mitochondrial dysfunction, exacerbating neuroinflammation in intracerebral hemorrhage
Abstract
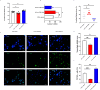
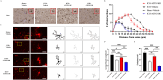
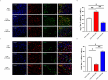
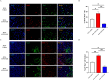
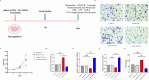
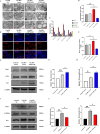
Microglial-mediated neuroinflammation is crucial in the pathophysiological mechanisms of secondary brain injury (SBI) following intracerebral hemorrhage (ICH). Mitochondria are central regulators of inflammation, influencing key pathways such as alternative splicing, and play a critical role in cell differentiation and function. Mitochondrial ATP synthase coupling factor 6 (ATP5J) participates in various pathological processes, such as cell proliferation, migration, and inflammation. However, the role of ATP5J in microglial activation and neuroinflammation post-ICH is poorly understood. This study aimed to investigate the effects of ATP5J on microglial activation and subsequent neuroinflammation in ICH and to elucidate the underlying mechanisms. We observed that ATP5J was upregulated in microglia after ICH. AAV9-mediated ATP5J overexpression worsened neurobehavioral deficits, disrupted the blood-brain barrier, and increased brain water content in ICH mice. Conversely, ATP5J knockdown ameliorated these effects. ATP5J overexpression also intensified microglial activation, neuronal apoptosis, and inflammatory responses in surrounding tissues post-ICH. ATP5J impaired microglial dynamics and reduced the proliferation and migration of microglia to injury sites. We used oxyhemoglobin (OxyHb) to stimulate BV2 cells and model ICH in vitro. Further in vitro studies showed that ATP5J overexpression enhanced OxyHb-induced microglial functional transformation. Mechanistically, ATP5J silencing reversed dynamin-related protein 1 (Drp1) and mitochondrial fission 1 protein (Fis1) upregulation in microglia post-OxyHb induction; reduced mitochondrial overdivision, excessive mitochondrial permeability transition pore opening, and reactive oxygen species production; restored normal mitochondrial ridge morphology; and partially restored mitochondrial respiratory electron transport chain activity. ATP5J silencing further alleviated OxyHb-induced mitochondrial dysfunction by regulating mitochondrial metabolism. Our results indicate that ATP5J is a key factor in regulating microglial functional transformation post-ICH by modulating mitochondrial dysfunction and metabolism, thereby positively regulate neuroinflammation. By inhibiting ATP5J, SBI following ICH could be prevented. Therefore, ATP5J could be a candidate for molecular and therapeutic target exploration to alleviate neuroinflammation post-ICH.
Keywords: ATP5J; intracerebral hemorrhage; microglia; mitochondrial reprogramming; secondary brain injury.
Copyright © 2024 Ren, Zhang, Li, Ji, Zhang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

