Circulating extracellular vesicles and neutrophil extracellular traps contribute to endothelial dysfunction in preeclampsia
- PMID: 39735539
- PMCID: PMC11671372
- DOI: 10.3389/fimmu.2024.1488127
Circulating extracellular vesicles and neutrophil extracellular traps contribute to endothelial dysfunction in preeclampsia
Abstract
Background: Preeclampsia (PE) is a pregnancy complication characterized by hypertension, proteinuria, endothelial dysfunction, and complement dysregulation. Placenta-derived extracellular vesicles (EVs), necessary in maternal-fetal communication, might contribute to PE pathogenesis. Moreover, neutrophil extracellular traps (NETs) play a pathogenic role in other complement-mediated pathologies, and their contribution in PE remains unexplored.
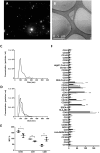
Materials and methods: EVs were isolated from PE (peEVs) and normotensive pregnant women sera. NETs were obtained incubating donor-pre-activated neutrophils with PE or control sera. Microvascular (HMEC) endothelial cells (ECs) were incubated with PE or control sera with or without (depleted sera) EVs or NETs, to assess changes in VCAM-1, ICAM-1, VE-cadherin, eNOS, VWF, ROS, and C5b-9 deposits. Results were expressed as fold increase vs. control.
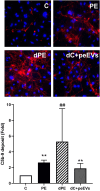
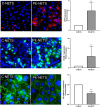
Results: VWF, VCAM-1, and ROS expression was significantly higher in cells exposed to PE sera vs. control (12.3 ± 8.1, 3.6 ± 2.3, and 1.8 ± 0.2, respectively, p < 0.05), though significantly lower in cells exposed to depleted PE (dPE) sera (6.1 ± 2.7, 0.7 ± 0.6, and 1.2 ± 0.1, respectively, vs. control, p < 0.05). EC exposure to depleted control sera supplemented with peEVs (dC+peEVs) significantly increased VWF, VCAM-1, and ROS compared to non-supplemented sera (4.5 ± 0.3, 2.8 ± 2.0, and 1.4 ± 0.2, respectively, p < 0.05). ICAM-1, VE-cadherin, and C5b-9 did not differ among groups. ECs incubated with PE-NETs increased VWF and VCAM-1 and decreased VE-cadherin expression vs. control (4 ± 1.6, 5.9 ± 1.2, and 0.5 ± 0.1, respectively, p < 0.05), and notably increased C5b-9 deposit (7.5 ± 2.9, p < 0.05). ICAM-1 and ROS did not differ.
Conclusions: Both circulating EVs and NETs from PE pregnant women exhibit a deleterious effect on ECs. Whereas EVs trigger a pro-oxidant and proinflammatory state, NETs potentiate the activation of the complement system, as already described in PE.
Keywords: complement membrane attack complex; endothelium; exosome; neutrophil activation; oxidative stress; pre-eclampsia.
Copyright © 2024 Ramos, Youssef, Molina, Torramadé-Moix, Martinez-Sanchez, Moreno-Castaño, Blasco, Guillén-Olmos, De Moner, Pino, Tortajada, Camacho, Borrell, Crovetto, Ramirez-Bajo, Ventura-Aguiar, Banon-Maneus, Rovira, Escolar, Carreras, Gratacos, Diaz-Ricart, Crispi and Palomo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

