Immunotherapy for lung adenocarcinoma patients with bone metastases: who really needs it
- PMID: 39735542
- PMCID: PMC11671745
- DOI: 10.3389/fimmu.2024.1457916
Immunotherapy for lung adenocarcinoma patients with bone metastases: who really needs it
Abstract
Background: Lung adenocarcinoma patients are often found to have developed bone metastases at the time of initial diagnosis. With the continuous development of technology, we have successfully entered the era of immunotherapy. This study aimed to determine the efficacy of immunotherapy in lung adenocarcinoma patients with bone metastases (LABM) through a multicenter retrospective analysis and to develop a novel tool to identify the population that could benefit most from immunotherapy.
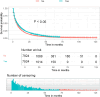
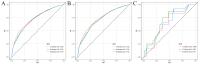
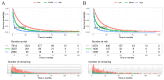
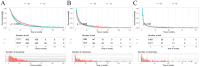
Methods: To assess the impact of immunotherapy on LABM in terms of overall survival, we used analytical tools such as Kaplan-Meier analysis, Log-ranch test, and propensity score matching (PSM) method. A predictive model for constructing overall survival was constructed using Cox regression modeling. Based on this, we developed a risk classification system depicting Kaplan-Meier curves for subgroup analysis to determine the optimal beneficiary population for immunotherapy in different risk subgroups.
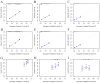
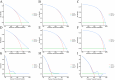
Results: A total of 20073 eligible patients were enrolled in this study, of whom 8010 did not receive immunotherapy, while 12063 patients received immunotherapy. After 1:1 PSM, 15848 patients were successfully coordinated, yielding a balanced cohort. Kaplan-Meier survival curves showed significantly enhanced overall survival (P < 0.001) in patients who received immunotherapy compared to those who did not. The results of Cox regression analyses showed that age, race, sex, primary site, immunotherapy, surgery, chemotherapy, brain metastasis, liver metastasis, lung metastasis, and marital status were independent prognostic factors. The area under the curve for all three cohorts was close to 0.7, indicating that the model was well-discriminating. The calibration curves further proved that the model had a high predictive accuracy. Decision curve analysis demonstrated that the model could achieve a high net clinical benefit. The risk classification system developed based on the model successfully screened the best beneficiary population for immunotherapy.
Conclusion: This study provides convincing evidence that immunotherapy provides a significant survival advantage for LABM. Secondly, the clinical tools constructed in this study can help clinicians identify the optimal population to benefit from immunotherapy in LABM, thus enabling precise treatment and avoiding the waste of medical resources and over-treatment of patients.
Keywords: SEER; bone metastases; immunotherapy; lung cancer; prognosis.
Copyright © 2024 Huang, Tong, Zhu, Yang, Chen and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Rebecca LS, Angela NG, Ahmedin J. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21830 - DOI
-
- Luis GP-A, Oscar J-V, Giannis SM, Enriqueta F, Niels R, Filippo DM, et al. Sacituzumab govitecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: the randomized, open-label phase III EVOKE-01 study. J Clin Oncol. (2024) 42(24):2860–72. doi: 10.1200/JCO.24.00733 - DOI - PMC - PubMed
-
- Rui Z, Zhi-Yu W, Yue-Hua L, Yao-Hong L, Shuai W, Wen-Xi Y, et al. Dynamic contrast-enhanced MRI to predict response to vinorelbine-cisplatin alone or with rh-endostatin in patients with non-small cell lung cancer and bone metastases: a randomised, double-blind, placebo-controlled trial. Lancet. (2016) 388(Suppl 1):S95. doi: 10.1016/S0140-6736(16)32022-0 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

