In vivo programmed myeloid cells expressing novel chimeric antigen receptors show potent anti-tumor activity in preclinical solid tumor models
- PMID: 39735543
- PMCID: PMC11671302
- DOI: 10.3389/fimmu.2024.1501365
In vivo programmed myeloid cells expressing novel chimeric antigen receptors show potent anti-tumor activity in preclinical solid tumor models
Abstract
Introduction: The approval of chimeric antigen receptor (CAR) T cell therapies for the treatment of B cell malignancies has fueled the development of numerous ex vivo cell therapies. However, these cell therapies are complex and costly, and unlike in hematological malignancies, outcomes with most T cell therapies in solid tumors have been disappointing. Here, we present a novel approach to directly program myeloid cells in vivo by administering novel TROP2 CAR mRNA encapsulated in lipid nanoparticles (LNPs).
Methods: The CAR comprises a TROP2 specific single-chain variable fragment (scFv) fused to a truncated CD89 which requires association with the FcRγ signal adapter to trigger myeloid-specific cell activation. The mRNA encoding the TROP2 CAR was encapsulated in LNPs. Co-immunoprecipitation, flow cytometry and enzyme-linked immunosorbent assay (ELISA) were used to measure CAR expression and functional activity in vitro. Anti-tumor efficacy of the TROP2 CAR mRNA/LNP was evaluated after intravenous administration in various murine tumor models.
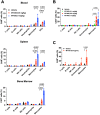
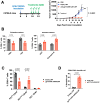
Results: In vitro, transient expression of the TROP2 CAR on monocytes triggers antigen-dependent cytotoxicity and cytokine release. In tumor bearing mice and cynomolgus monkeys, the TROP2 CAR mRNA/LNP are primarily expressed by myeloid cells. In a mouse xenograft model, intravenous administration of TROP2 CAR mRNA/LNP results in tumor growth inhibition and in a B16/F10-OVA immunocompetent melanoma mouse model, anti-tumor efficacy of a gp75-specific CAR correlates with increased number of activated T cells, activation of dendritic cells and a humoral response against B16/F10-OVA melanoma tumors.
Discussions: These findings demonstrate that myeloid cells can be directly engineered in vivo to kill tumor cells and orchestrate an adaptive immune response and guide clinical studies for the treatment of solid tumors.
Keywords: RNA therapeutics; cancer therapy; cell therapy; lipid nanoparticles; myeloid cells.
Copyright © 2024 Argueta, Wang, Zhao, Diwanji, Gorgievski, Cochran, Grudzien-Nogalska, D’Alessandro, McCreedy, Prod’homme, Hofmeister, Ding and Getts.
Conflict of interest statement
All authors were employed by Myeloid Therapeutics, Inc.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

