Frequency, characteristics, and immunological accompaniments of ataxia in anti-NMDAR antibody-associated encephalitis
- PMID: 39735552
- PMCID: PMC11681429
- DOI: 10.3389/fimmu.2024.1500904
Frequency, characteristics, and immunological accompaniments of ataxia in anti-NMDAR antibody-associated encephalitis
Abstract
Introduction: Very rarely, adult NMDAR antibody-associated encephalitis (NMDAR-E) leads to persistent cerebellar atrophy and ataxia. Transient cerebellar ataxia is common in pediatric NMDAR-E. Immune-mediated cerebellar ataxia may be associated with myelin oligodendrocyte glycoprotein (MOG), aquaporin-4 (AQP-4), kelch-like family member 11 (KLHL11), and glutamate kainate receptor subunit 2 (GluK2) antibodies, all of which may co-occur in NMDAR-E. Here, we aimed to investigate the frequency, long-term outcome, and immunological concomitants of ataxia in NMDAR-E.
Methods: In this observational study, patients with definite NMDAR-E with a follow-up of >12 months were recruited from the GENERATE registry. Cases with documented ataxia were analyzed in detail.
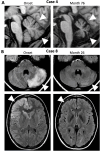
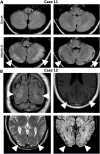
Results: In 12 of 62 patients (19%), ataxia was documented. Bilateral cerebellar ataxia without additional focal CNS findings was found in four (one child and three adults); one of these was previously reported as a case with persistent cerebellar atrophy and ataxia. Two patients with bilateral cerebellar ataxia had additional focal neurological symptoms, optic neuritis and facial palsy. Two patients developed hemiataxia: one with diplopia suggesting brainstem dysfunction and the other probably resulting from cerebellar diaschisis due to contralateral status epilepticus. In all but the one developing cerebellar atrophy, cerebellar ataxia was transient and not associated with a worse long-term outcome. In all five patients with cerebellar ataxia tested, MOG, AQP-4, GluK2, and KLHL11 antibodies were negative. In two additional patients negative for both MOG and AQP-4 antibodies, ataxia was sensory and explained by cervical myelitis as part of multiple sclerosis (MS) manifesting temporal relation to NMDAR-E. One of the patients with bilateral ataxia with focal neurological deficits was also diagnosed with MS upon follow-up. Finally, in two patients, ataxia was explained by cerebral hypoxic damage following circulatory failure during an ICU stay with severe NMDAR-E.
Discussion: Ataxia of different types is quite common in NMDAR-E. Cerebellar ataxia in NMDAR-E is mostly transient. NMDAR-E followed by persistent ataxia and cerebellar atrophy is very rare. Cerebellar ataxia in NMDAR-E may not be explained by concomitant KLHL11, MOG, AQP-4, or GluK2 autoimmunity. Of note, ataxia in NMDAR-E may result from treatment complications and, most interestingly, from MS manifesting in temporal association with NMDAR-E.
Keywords: MOG antibody; NMDAR-encephalitis; aquaporin-4 antibody; ataxia; cerebellum; multiple sclerosis; outcome.
Copyright © 2024 Jesse, Riemann, Schneider, Ringelstein, Melzer, Vogel, Pfeffer, Friese, Sühs, Hudasch, Schwenkenbecher, Günther, Geis, Wickel, Lesser, Kather, Leypoldt, Dargvainiene, Markewitz, Wandinger, Thaler, Kuchling, Wurdack, Sabater, Finke and Lewerenz.
Conflict of interest statement
MF received honoraria as speaker and for consultation from Alexion, Biogen, Kyverna, Lundbeck, Merck KGaA, Novartis, Roche and Sudo Biosciences, as well as from the Gemeinnützige Hertie-Stiftung and Wings for Life. His research is funded by the German Federal Ministry of Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Landesforschungsförderung Hamburg, Gemeinnützige Hertie-Stiftung, Else Kröner-Fresenius-Stiftung, Deutsche Multiple Sklerose Gesellschaft, Fritz-Thyssen-Stiftung, Werner-Otto-Stiftung, Walter und Ilse Rose-Stiftung, Stiftung zur Bekämpfung neuroviraler Erkrankungen and Research Fund of the University Medical Center Hamburg-Eppendorf; AG received speaker’s honoraria from Boehringer Ingelheim, Daichii Sankyo, Pfizer, Merz, Shionogi, Occlutech and Ipsen Pharma as well as research grants from MERZ and Ipsen Pharma; FL discloses speaker honoraria from Grifols, Teva, Biogen, Bayer, Roche, Novartis, Fresenius, travel funding from Merck, Grifols and Bayer and serving on advisory boards for Roche, Biogen and Alexion. His research FL is supported by German Ministry of Education and Research (BMBF), 01GM1908A und 01GM2208A, CONNECT-GENERATE, by E-Rare Joint Transnational research support ERA-Net, LE3064/2-1, Stiftung Pathobiochemie of the German Society for Laboratory Medicine and HORIZON MSCA 2022 Doctoral Network 101119457 — IgG4-TREAT; JL reports travel honoraria and speaker’s fees from the Cure Huntington’s Disease Initiative (CHDI), the Movement Disorders Society as the German Society for Cerebrospinal Fluid Diagnostic and Clinical Neurochemistry (DGLN), Alexion, Biogen, Sanofi, Teva, Merck, Novartis, Janssen, Fujirebio, Roche, and Neuraxpharm. His institution received financial compensation for clinical trials with JL as principal investigator from CHDI and SOM Biotech. He is member of the executive board of the DGLN. He received research funding from the German Federal Ministry of Education and Research (BMBF), CONNECT-GENERATE, 01GM1908B und 01GM2208B; and the Boehringer Ingelheim University Biocenter; NM has received honoraria for lecturing and travel expenses for attending meetings from Biogen Idec, GlaxoSmith Kline, Teva, Novartis Pharma, Bayer Healthcare, Genzyme, Alexion Pharmaceuticals, Fresenius Medical Care, Diamed, UCB Pharma, AngeliniPharma, BIAL and Sanofi-Aventis, has received royalties for consulting from UCB Pharma, Alexion Pharmaceuticals; and Sanofi-Aventis and has received financial research support from Euroimmun, Fresenius Medical Care, Diamed, Alexion Pharmaceuticals, and Novartis Pharma; MR received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, Horizon/Amgen; and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, Roche, Horizon, Alexion; and Merck, none related to this study; K-WS reports honoraria for lectures or travel reimbursements for attending meetings from Biogen, Merck, Mylan, Roche, Bavarian Nordic, Viatris and Bristol-Myers Squibb as well as research support from Bristol-Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, et al. . Fluorodeoxyglucose Positron Emission Tomography in Anti-N-Methyl-D-Aspartate Receptor Encephalitis: Distinct Pattern of Disease. J Neurol Neurosurg Psychiatry. (2012) 83(7):681–6. doi: 10.1136/jnnp-2011-301969 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

