Quorum sensing signals of the grapevine crown gall bacterium, Novosphingobium sp. Rr2-17: use of inducible expression and polymeric resin to sequester acyl-homoserine lactones
- PMID: 39735558
- PMCID: PMC11674143
- DOI: 10.7717/peerj.18657
Quorum sensing signals of the grapevine crown gall bacterium, Novosphingobium sp. Rr2-17: use of inducible expression and polymeric resin to sequester acyl-homoserine lactones
Abstract
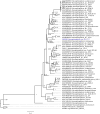
Background: A grapevine crown gall tumor strain, Novosphingobium sp. strain Rr2-17 was previously reported to accumulate copious amounts of diverse quorum sensing signals during growth. Genome sequencing identified a single luxI homolog in strain Rr2-17, suggesting that it may encode for a AHL synthase with broad substrate range, pending functional validation. The exact identity of the complete suite of AHLs formed by novIspR1 is largely unknown.
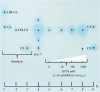
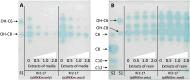
Methods: This study validates the function of novIspR1 through inducible expression in Escherichia coli and in the wild-type parental strain Rr2-17. We further enhanced the capture of acyl homoserine lactone (AHL) signals produced by novIspR1 using polymeric resin XAD-16 and separated the AHLs by one- and two-dimensional thin layer chromatography followed by detection using AHL-dependent whole cell biosensor strains. Lastly, the complete number of AHLs produced by novIspR1 in our system was identified by LC-MS/MS analyses.
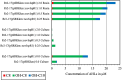
Results: The single LuxI homolog of N. sp. Rr2-17, NovIspR1, is able to produce up to eleven different AHL signals, including AHLs: C8-, C10-, C12-, C14-homoserine lactone (HSL) as well as AHLs with OH substitutions at the third carbon and includes 3-OH-C6-, 3-OH-C8-, 3-OH-C10-, 3-OH-C12- and 3-OH-C14-HSL. The most abundant AHL produced was identified as 3-OH-C8-HSL and isopropyl-D-1-thiogalactopyranoside (IPTG) induction of novIspR1 expression in wild type parental Rr2-17 strain increased its concentration by 6.8-fold when compared to the same strain with the vector only control plasmid. Similar increases were identified with the next two most abundant AHLs, 3-OH-C10- and unsubstituted C8-HSL. The presence of 2% w/v of XAD-16 resin in the growth culture bound 99.3 percent of the major AHL (3-OH-C8-HSL) produced by IPTG-induced overexpression of novIspR1 in Rr2-17 strain. This study significantly adds to our understanding of the AHL class of quorum sensing system in a grapevine crown gall tumor associated Novosphingobium sp. Rr2-17 strain. The identity of nine AHL signals produced by this bacterium will provide a framework to identify the specific function(s) of the AHL-mediated quorum-sensing associated genes in this bacterium.
Keywords: Acyl-homoserine lactones; Agrobacterium vitis tumor; Grapevine crown gall tumor; Inducible expression; NovI; Novosphingobium. sp.; Quorum sensing; Resin.
© 2024 Gan et al.
Conflict of interest statement
The authors declare that they have no competing interests. Han Ming Gan is employed by Patriot Biotech Sdn Bhd.
Figures

Similar articles
-
Genome sequence of Novosphingobium sp. strain Rr 2-17, a nopaline crown gall-associated bacterium isolated from Vitis vinifera L. grapevine.J Bacteriol. 2012 Sep;194(18):5137-8. doi: 10.1128/JB.01159-12. J Bacteriol. 2012. PMID: 22933764 Free PMC article.
-
Human TRPV1 and TRPA1 are receptors for bacterial quorum sensing molecules.J Biochem. 2022 Jan 7;170(6):775-785. doi: 10.1093/jb/mvab099. J Biochem. 2022. PMID: 34557892
-
Characterization of quorum sensing genes and N-acyl homoserine lactones in Citrobacter amalonaticus strain YG6.Gene. 2019 Feb 5;684:58-69. doi: 10.1016/j.gene.2018.10.031. Epub 2018 Oct 12. Gene. 2019. PMID: 30321658
-
Molecular Mechanisms and Applications of N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Bacteria.Molecules. 2022 Nov 4;27(21):7584. doi: 10.3390/molecules27217584. Molecules. 2022. PMID: 36364411 Free PMC article. Review.
-
An evolving perspective on the Pseudomonas aeruginosa orphan quorum sensing regulator QscR.Front Cell Infect Microbiol. 2014 Oct 28;4:152. doi: 10.3389/fcimb.2014.00152. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25389523 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

