Onset and long-term duration of immunity provided by a single vaccination with recombinant a Marek's disease virus with REV-LTR insertion
- PMID: 39735581
- PMCID: PMC11681624
- DOI: 10.3389/fvets.2024.1510834
Onset and long-term duration of immunity provided by a single vaccination with recombinant a Marek's disease virus with REV-LTR insertion
Abstract
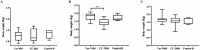
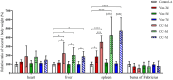
Marek's Disease (MD), caused by Marek's disease virus (MDV), is a highly contagious lymphoproliferative disease in poultry. Despite the fact that MD has been effectively controlled by vaccines, the virulence of field isolates of MDV has continued to evolve, becoming more virulent under the immune pressure of vaccines. Our previous research has confirmed that the recombinant rMDV strain with REV-LTR insertion can be used as a live attenuated vaccine candidate. The aim of this research was to evaluate the onset and duration of immunity of the rMDV strain through two experiments. In both experiments, 1-day-old SPF chickens were vaccinated subcutaneously with the rMDV strain at a dose of 3,000 Plaque Formation Unit (PFU) per chick in 0.2 mL of the MD diluent. Then, in Experimental design 1, the chicks in the groups Vac-3d/CC-3d, Vac-5d/CC-5d, and Vac-7d/CC-7d were challenged separately with 500 PFU vvMDV strain MD5 at 3 days, 5 days, and 7 days after vaccination; in Experimental design 2, the chicks in group Vac-60d/CC-60d, Vac-120d/CC-120d, and Vac-180d/CC-180d were challenged at 60 days, 120 days, and 180 days after vaccination. The clinical symptoms and weight gain of chickens in each group were observed and recorded. The results showed that the rMDV strain with REV-LTR insertion provides protection starting from 3 days of age and achieves good immune effects at 5 days of age after 1-day-old immunization, and the immunization duration can reach for at least 180 days. Given age-related resistance, it can be confirmed that our vaccine can actually provide lifelong immunity. This study provides valuable insights into the onset and duration of immunity of the rMDV strain, which will provide a basis for the development and improvement of MD vaccines.
Keywords: Marek’s disease virus; REV-LTR; duration of immunity; onset of immunity; vaccine.
Copyright © 2024 Dai, Song, Tan, Sun, Tang, Qu, Liao, Qiu and Ding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Morrow C., Fehler F. (2024). Marek’s disease: A worldwide problem. ScienceDirect. (2004) 12:49–61. doi: 10.3109/10408418509104432 - DOI
-
- Osterrieder K, Vautherot J-F. 3 - the genome content of Marek’s disease-like viruses In: Davison F, Nair V, editors. Marek’s Disease. Oxford: Academic Press; (2004). 17–31.
-
- Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, Dutilh BE, et al. Changes to virus taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses (2019). Arch Virol. (2019) 164:2417–29. doi: 10.1007/s00705-019-04306-w - DOI - PubMed
LinkOut - more resources
Full Text Sources

