The effects of camel milk in systemic inflammation and oxidative stress of cigarette smoke-induced chronic obstructive pulmonary disease model in rat
- PMID: 39735585
- PMCID: PMC11673985
- DOI: 10.3389/fvets.2024.1464432
The effects of camel milk in systemic inflammation and oxidative stress of cigarette smoke-induced chronic obstructive pulmonary disease model in rat
Abstract
Background: The effects of camel milk in inflammation and systemic oxidative stress of cigarette smoke (CS)-induced chronic obstructive pulmonary disease (COPD) associated with small airway inflammation in rats were investigated.
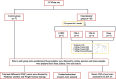
Methods: 35 male Wistar rats were randomly divided into five groups: (a) control, (b) CS-exposed rats, c and (d) CS-exposed rats treated with the 4 and 8 mL/kg camel milk, and (e) CS-exposed rats treated with 1 mg/kg dexamethasone.
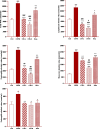
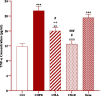
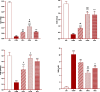
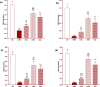
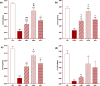
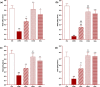
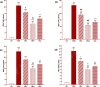
Results: Total and differential WBC counts, serum level of TNF-α and malondialdehyde (MDA) level in serum and homogenized tissues of the heart, kidney, liver, and testicle were significantly increased, but catalase (CAT), superoxide dismutase (SOD) and thiol levels were significantly decreased in CS-exposed rats (p < 0.01 to p < 0.001). Treatment with dexamethasone and both doses of camel milk improved all measured variables compared to the COPD group (p < 0.05 to p < 0.001). The improvements of most variables in the treated group with high dose of camel milk were higher than the effect of dexamethasone (p < 0.05 to p < 0.001). These findings suggest that camel milk has a therapeutic potential for treating systemic oxidative stress and inflammatory induced by CS.
Conclusion: Therefore, camel milk might be effective in attenuating the effects of CS-induced systemic inflammation and oxidative stress.
Keywords: COPD; camel milk; cytokine; oxidative stress; pulmonary disease.
Copyright © 2024 Behrouz, Mohammadi, Sarir and Boskabady.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

