SBL-JP-0004: A promising dual inhibitor of JAK2 and PI3KCD against gastric cancer
- PMID: 39735669
- PMCID: PMC11671411
- DOI: 10.32604/or.2024.055677
SBL-JP-0004: A promising dual inhibitor of JAK2 and PI3KCD against gastric cancer
Abstract
Background: Gastric cancer (GC) remains a global health burden and is often characterized by heterogeneous molecular profiles and resistance to conventional therapies. The phosphoinositide 3-kinase and PI3K and Janus kinase (JAK) signal transducer and activator of transcription (JAK-STAT) pathways play pivotal roles in GC progression, making them attractive targets for therapeutic interventions.
Methods: This study applied a computational and molecular dynamics simulation approach to identify and characterize SBL-JP-0004 as a potential dual inhibitor of JAK2 and PI3KCD kinases. KATOIII and SNU-5 GC cells were used for in vitro evaluation.
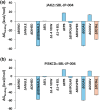
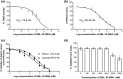
Results: SBL-JP-0004 exhibited a robust binding affinity for JAK2 and PI3KCD kinases, as evidenced by molecular docking scores and molecular dynamics simulations. Binding interactions and Gibbs binding free energy estimates confirmed stable and favorable interactions with target proteins. SBL-JP-0004 displayed an half-maximal inhibitory concentration (IC50) value of 118.9 nM against JAK2 kinase and 200.9 nM against PI3KCD enzymes. SBL-JP-0004 exhibited potent inhibition of cell proliferation in KATOIII and SNU-5 cells, with half-maximal growth inhibitory concentration (GI50) values of 250.8 and 516.3 nM, respectively. A significant elevation in the early phase apoptosis (28.53% in KATOIII cells and 26.85% in SNU-5 cells) and late phase apoptosis (17.37% in KATOIII cells and 10.05% in SNU-5 cells) were observed with SBL-JP-0004 treatment compared to 2.1% and 2.83% in their respective controls.
Conclusion: The results highlight SBL-JP-0004 as a promising dual inhibitor targeting JAK2 and PI3KCD kinases for treating GC and warrant further preclinical and clinical investigations to validate its utility in clinical settings.
Keywords: Dual inhibition; Gastric cancer (GC); JAK2; Molecular docking; Molecular dynamics simulation; PI3KCD; SBL-JP-0004.
© 2025 The Author.
Conflict of interest statement
The author declares no conflicts of interest to report regarding the present study.
Figures





Similar articles
-
Dual Targeting of MEK1 and Akt Kinase Identified SBL-027 as a Promising Lead Candidate to Control Cell Proliferations in Gastric Cancer.Biotechnol Appl Biochem. 2025 Aug;72(4):1006-1017. doi: 10.1002/bab.2716. Epub 2025 Jan 7. Biotechnol Appl Biochem. 2025. PMID: 39777426
-
Astaxanthin-loaded PLGA nanoparticles inhibit survival of MKN-45 gastric cancer cell line by modulating JAK2/STAT3/mTOR/PI3K pathway.BMC Cancer. 2025 Jan 9;25(1):44. doi: 10.1186/s12885-024-13401-4. BMC Cancer. 2025. PMID: 39780129 Free PMC article.
-
Discovery and rational design of 2-aminopyrimidine-based derivatives targeting Janus kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3).Bioorg Chem. 2020 Nov;104:104361. doi: 10.1016/j.bioorg.2020.104361. Epub 2020 Oct 9. Bioorg Chem. 2020. PMID: 33142418
-
Inhibitors of JAK2 and JAK3: an update on the patent literature 2010 - 2012.Expert Opin Ther Pat. 2013 Apr;23(4):449-501. doi: 10.1517/13543776.2013.765862. Epub 2013 Feb 1. Expert Opin Ther Pat. 2013. PMID: 23367873 Review.
-
Progress in the preclinical discovery and clinical development of class I and dual class I/IV phosphoinositide 3-kinase (PI3K) inhibitors.Curr Med Chem. 2011;18(18):2686-714. doi: 10.2174/092986711796011229. Curr Med Chem. 2011. PMID: 21649578 Free PMC article. Review.
Cited by
-
CEG-0598, a novel dual inhibitor of EGFR and C5aR demonstrates in vitro anticancer and antimetastatic activity in prostate cancer cells.Discov Oncol. 2025 May 9;16(1):710. doi: 10.1007/s12672-025-02574-4. Discov Oncol. 2025. PMID: 40343625 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
