TMED3 promotes prostate cancer via FOXO1a and FOXO3a phosphorylation
- PMID: 39735675
- PMCID: PMC11671412
- DOI: 10.32604/or.2024.048054
TMED3 promotes prostate cancer via FOXO1a and FOXO3a phosphorylation
Abstract
Background: Transmembrane emp24 trafficking protein 3 (TMED3) is associated with the development of several tumors; however, whether TMED3 regulates the progression of prostate cancer remains unclear.
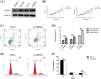
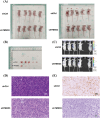
Materials and methods: Short hairpin RNA was performed to repress TMED3 in prostate cancer cells (DU145 cells) and in a prostate cancer mice model to determine its function in prostate cancer in vitro and in vivo.
Results: In the present study, we found that TMED3 was highly expressed in prostate cancer cells. In vitro, shTMED3 treatment suppressed the proliferation, invasion, and migration and promoted the apoptosis of DU145 cells. Additionally, the Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis showed a strong correlation between TMED3 and forkhead box O transcription factor (FOXO) pathway. Furthermore, TMED3 inhibition efficiently decreased FOXO1a and FOXO3a phosphorylation. In vivo, TMED3 downregulation suppressed the apoptosis, growth, and metastasis of prostate cancer cells via FOXO1a and FOXO3a.
Conclusion: The present findings show that TMED3 participates in the regulation of prostate cancer progression via FOXO1a and FOXO3a phosphorylation, thereby revealing a novel mechanism underlying prostate cancer development and suggesting that TMED3 inhibition may serve as a novel strategy for prostate cancer treatment.
Keywords: Apoptosis; Proliferation; Prostate cancer; Transmembrane emp24 trafficking protein 3 (TMED3); forkhead box O transcription factor (FOXO).
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest to report regarding the present study.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
