Nutraceuticals silybin B, resveratrol, and epigallocatechin-3 gallate-bind to cardiac muscle troponin to restore the loss of lusitropy caused by cardiomyopathy mutations in vitro, in vivo, and in silico
- PMID: 39735723
- PMCID: PMC11672104
- DOI: 10.3389/fphys.2024.1489439
Nutraceuticals silybin B, resveratrol, and epigallocatechin-3 gallate-bind to cardiac muscle troponin to restore the loss of lusitropy caused by cardiomyopathy mutations in vitro, in vivo, and in silico
Abstract
Introduction: Adrenergic activation of protein kinase A (PKA) in cardiac muscle targets the sarcolemma, sarcoplasmic reticulum, and contractile apparatus to increase contractile force and heart rate. In the thin filaments of the contractile apparatus, cardiac troponin I (cTnI) Ser22 and Ser23 in the cardiac-specific N-terminal peptide (NcTnI: residues 1 to 32) are the targets for PKA phosphorylation. Phosphorylation causes a 2-3 fold decrease of affinity of cTn for Ca2+ associated with a higher rate of Ca2+ dissociation from cTnC leading to a faster relaxation rate of the cardiac muscle (lusitropy). Cardiomyopathy-linked mutations primarily affect Ca2+ regulation or the PKA-dependent modulatory system, such that Ca2+-sensitivity becomes independent of phosphorylation level (uncoupling) and this could be sufficient to induce cardiomyopathy. A drug that could restore the phosphorylation-dependent modulation of Ca2+-sensitivity could have potential for treatment of these pathologies. We have found that a number of small molecules, including silybin B, resveratrol and EGCG, can restore coupling in single filament assays.
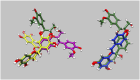
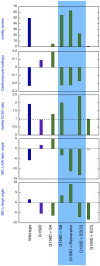
Methods: We did molecular dynamics simulations (5x1500ns for each condition) of the unphosphorylated and phosphorylated cardiac troponin core with the G159D DCM mutation in the presence of the 5 ligands and analysed the effects on several dynamic parameters. We also studied the effect of the ligands on the contractility of cardiac muscle myocytes with ACTC E99K and TNNT2 R92Q mutations in response to dobutamine.
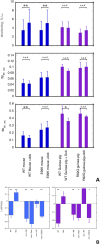
Results: Silybin B, EGCG and resveratrol restored the phosphorylation-induced change in molecular dynamics to wild-type values, whilst silybin A, an inactive isomer of silybin B, and Epicatechin gallate, an EGCG analogue that does not recouple, did not. We analysed the atomic-level changes induced by ligand binding to explain recoupling. Mutations ACTC E99K and TNNT2 R92Q blunt the increased relaxation speed response to β1 adrenergic stimulation of cardiac myocytes and we found that resveratrol, EGCG and silybin B could restore the β1 adrenergic response, whereas silybin A did not.
Discussion: The uncoupling phenomenon caused by cardiomyopathy-related mutations and the ability of small molecules to restore coupling in vitro and lusitropy in myocytes is observed at the cellular, molecular and atomistic levels therefore, restoring lusitropy is a suitable target for treatment. Further research on compounds that restore lusitropy is thus indicated as treatments for genetic cardiomyopathies. Further molecular dynamics simulations could define the specific properties needed for recoupling and allow for the prediction and design of potential new drugs.
Keywords: EGCG; PKA phosphorylation; cardiomyopathy; lusitropy; molecular dynamics simulation; myocyte contraction; silybin; troponin (cTnI).
Copyright © 2024 Yang, Sheehan, Messer, Tsui, Sparrow, Redwood, Kren, Gould and Marston.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bayly C. I., Cieplak P., Cornell W., Kollman P. A. (1993). A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97 (40), 10269–10280. 10.1021/j100142a004 - DOI
-
- Becke A. D. (1993). Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98 (7), 5648–5652. 10.1063/1.464913 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

