Challenges and Opportunities in Targeting the Complex Pancreatic Tumor Microenvironment
- PMID: 39735733
- PMCID: PMC11670921
- DOI: 10.1200/OA-24-00050
Challenges and Opportunities in Targeting the Complex Pancreatic Tumor Microenvironment
Abstract
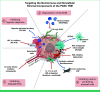
Pancreatic ductal adenocarcinoma (PDAC) is the third leading cause of cancer-related deaths with a 5-year survival rate of 13%. Surgical resection remains the only curative option as systemic therapies offer limited benefit. Poor response to chemotherapy and immunotherapy is due, in part, to the dense stroma and heterogeneous tumor microenvironment (TME). Opportunities to target the PDAC stroma may increase the effectiveness of existing or novel therapies. Current strategies targeting the stromal compartment within the PDAC TME primarily focus on degrading extracellular matrix or inhibiting stromal cell activity, angiogenesis, or hypoxic responses. In addition, extensive work has attempted to use immune targeting strategies to improve clinical outcomes. Preclinically, these strategies show promise, especially with the ability to alter the tumor ecosystem; however, when translated to the clinic, most of these trials have failed to improve overall patient outcomes. In this review, we catalog the heterogenous elements of the TME and discuss the potential of combination therapies that target the heterogeneity observed in the TME between patients and how molecular stratification could improve responses to targeted and combination therapies.
© 2024 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to https://ascopubs.org/authors. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Shaun M. Goodyear Consulting or Advisory Role: PDX Pharmacy Jonathan R. Brody Stock and Other Ownership Interests: Perthera, Faster Better Media Consulting or Advisory Role: Rimon Law Research Funding: Code Biotherapeutics (Inst) Other Relationship: Editor-in-Chief for Springer and Taylor No other potential conflicts of interest were reported. Jonathan R. Brody Stock and Other Ownership Interests: Perthera, Faster Better Media Consulting or Advisory Role: Rimon Law Research Funding: Code Biotherapeutics (Inst) Other Relationship: Editor-in-Chief for Springer and Taylor No other potential conflicts of interest were reported. Jonathan R. Brody Stock and Other Ownership Interests: Perthera, Faster Better Media Consulting or Advisory Role: Rimon Law Research Funding: Code Biotherapeutics (Inst) Other Relationship: Editor-in-Chief for Springer and Taylor No other potential conflicts of interest were reported.
Figures

References
-
- Sideras K, Braat H, Kwekkeboom J, et al. : Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 40:513-522, 2014 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
