Comparison analysis between standard polysomnographic data and in-ear-electroencephalography signals: a preliminary study
- PMID: 39735738
- PMCID: PMC11672114
- DOI: 10.1093/sleepadvances/zpae087
Comparison analysis between standard polysomnographic data and in-ear-electroencephalography signals: a preliminary study
Abstract
Study objectives: Polysomnography (PSG) currently serves as the benchmark for evaluating sleep disorders. Its discomfort makes long-term monitoring unfeasible, leading to bias in sleep quality assessment. Hence, less invasive, cost-effective, and portable alternatives need to be explored. One promising contender is the in-ear-electroencephalography (EEG) sensor. This study aims to establish a methodology to assess the similarity between the single-channel in-ear-EEG and standard PSG derivations.
Methods: The study involves 4-hour signals recorded from 10 healthy subjects aged 18-60 years. Recordings are analyzed following two complementary approaches: (1) a hypnogram-based analysis aimed at assessing the agreement between PSG and in-ear-EEG-derived hypnograms; and (2) a feature- and analysis-based on time- and frequency-domain feature extraction, unsupervised feature selection, and definition of Feature-based Similarity Index via Jensen-Shannon Divergence (JSD-FSI).
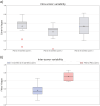
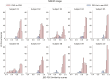
Results: We find large variability between PSG and in-ear-EEG hypnograms scored by the same sleep expert according to Cohen's kappa metric, with significantly greater agreements for PSG scorers than for in-ear-EEG scorers (p < .001) based on Fleiss' kappa metric. On average, we demonstrate a high similarity between PSG and in-ear-EEG signals in terms of JSD-FSI-0.79 ± 0.06-awake, 0.77 ± 0.07-nonrapid eye movement, and 0.67 ± 0.10-rapid eye movement-and in line with the similarity values computed independently on standard PSG channel combinations.
Conclusions: In-ear-EEG is a valuable solution for home-based sleep monitoring; however, further studies with a larger and more heterogeneous dataset are needed.
Keywords: in-ear-EEG; machine learning; multisource-scored sleep databases; sleep staging; sleep wearables.
© The Author(s) 2024. Published by Oxford University Press on behalf of Sleep Research Society.
Conflict of interest statement
SUPSI authors are responsible for the research, and they conducted the analysis independently. IDUN Company representative contribution was in offering detailed information about the device and the dataset collected in a previous study. This study has been previously disseminated as a preprint on arXiv.
Figures






Similar articles
-
Automatic sleep staging using ear-EEG.Biomed Eng Online. 2017 Sep 19;16(1):111. doi: 10.1186/s12938-017-0400-5. Biomed Eng Online. 2017. PMID: 28927417 Free PMC article.
-
Hearables: Automatic Overnight Sleep Monitoring With Standardized In-Ear EEG Sensor.IEEE Trans Biomed Eng. 2020 Jan;67(1):203-212. doi: 10.1109/TBME.2019.2911423. Epub 2019 Apr 22. IEEE Trans Biomed Eng. 2020. PMID: 31021747
-
The visual scoring of sleep and arousal in infants and children.J Clin Sleep Med. 2007 Mar 15;3(2):201-40. J Clin Sleep Med. 2007. PMID: 17557427 Review.
-
Expert-level automated sleep staging of long-term scalp electroencephalography recordings using deep learning.Sleep. 2020 Nov 12;43(11):zsaa112. doi: 10.1093/sleep/zsaa112. Sleep. 2020. PMID: 32478820 Free PMC article.
-
The Visual Scoring of Sleep in Infants 0 to 2 Months of Age.J Clin Sleep Med. 2016 Mar;12(3):429-45. doi: 10.5664/jcsm.5600. J Clin Sleep Med. 2016. PMID: 26951412 Free PMC article. Review.
References
-
- Stochholm A, Mikkelsen K, and Kidmose P.. Automatic sleep stage classification using ear-EEG. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2016: 4751–4754. doi: https://doi.org/10.1109/EMBC.2016.7591789. 2016 - DOI - PubMed
-
- Tabar YR, Mikkelsen KB, Shenton N, et al.At-home sleep monitoring using generic ear-EEG. Front Neurosci. 2023;17:987578. doi: https://doi.org/10.3389/fnins.2023.987578 - DOI - PMC - PubMed
-
- Zibrandtsen I, Kidmose P, Otto M, Ibsen J, Kjaer T.. Case comparison of sleep features from ear-EEG and scalp-EEG. Sleep Sci. 2016;9:69–72. doi: https://doi.org/10.1016/j.slsci.2016.05.006 - DOI - PMC - PubMed
-
- Berry RB, Brooks R, Gamaldo CE, et al.The AASM manual for the scoring of sleep and associated events. Rules Terminol Tech Specific Darien Illinois Am Acad Sleep Med. 2012;176:2012.
-
- Fiorillo L, Puiatti A, Papandrea M, et al.Automated sleep scoring: a review of the latest approaches. Sleep Med Rev. 2019;48:101204. doi: https://doi.org/10.1016/j.smrv.2019.07.007 - DOI - PubMed

