Acute Decompensated Valvular Disease in the Intensive Care Unit
- PMID: 39735779
- PMCID: PMC11681797
- DOI: 10.1016/j.jacadv.2024.101402
Acute Decompensated Valvular Disease in the Intensive Care Unit
Abstract
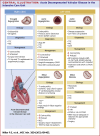
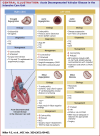
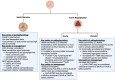
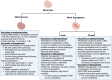
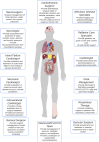
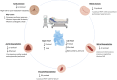
Acute decompensated valvular disease encompasses a group of complex and challenging conditions, which are often the primary reason for admission to the cardiac intensive care unit and can also complicate the management of other primary cardiac disorders. Critically ill patients with valvular disease also present unique diagnostic and management challenges. Historically, medical and percutaneous interventional therapies have been limited and surgery was the only definitive treatment; however, surgical risk can at times be prohibitive. High-quality evidence to direct management of acute valvular disorders in this population is lacking and societal guidelines largely do not address treatment options for critically ill patients with decompensated valvular disease. In this review, we discuss the clinical presentation and epidemiology of commonly encountered valvular diseases in the modern cardiac intensive care unit, highlight key pathophysiology, detail gaps in evidence, describe the pivotal role of multidisciplinary Heart Teams, and provide guidance for management.
Keywords: cardiac intensive care unit; cardiogenic shock; valvular disease.
© 2024 The Authors.
Conflict of interest statement
Dr Rali has received consulting/speaking honorarium from Analog Devices, Abiomed, Caretaker Medical, Vectorious, Volumetrix, and Zoll. Dr Bhatt has received consulting fees for programs sponsored by Sanofi. Dr Grubb is a speaker, proctor, and principal investigator for Edwards Lifesciences; is a speaker, proctor, and advisory board member for Boston Scientific; is a speaker, proctor, principal investigator, advisory board member, and national principal investigator for Medtronic; and her employer has research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. Dr Morrow has received research grant support to TIMI Study Group through Brigham and Women’s Hospital from Abbott Laboratories, Abiomed, Amgen, Anthos Therapeutics, Arca Biopharma, AstraZeneca, Daiichi-Sankyo, Intarcia, Janssen, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Regeneron, Roche, Siemens, and Zora Biosciences; and consulting fees from Abbott Laboratories, Arca Biopharma, InCarda, Inflammatix, Merck, Novartis, and Roche Diagnostics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Bohula E.A., Katz J.N., van Diepen S., et al. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the critical care Cardiology trials network prospective North American multicenter registry of cardiac critical illness. JAMA Cardiol. 2019;4:928–935. - PMC - PubMed
-
- Parlow S., Weng W., Di Santo P., et al. Significant valvular dysfunction and outcomes in cardiogenic shock: insights from the randomized DOREMI trial. Can J Cardiol. 2022;38:1211–1219. - PubMed
-
- Steffen J., Stocker A., Scherer C., et al. Emergency transcatheter aortic valve implantation for acute heart failure due to severe aortic stenosis in critically ill patients with or without cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2022;11:877–886. - PubMed
-
- Masha L., Vemulapalli S., Manandhar P., et al. Demographics, procedural characteristics, and clinical outcomes when cardiogenic shock precedes TAVR in the United States. JACC Cardiovasc Interv. 2020;13:1314–1325. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

