Molecular Diagnosis of Helicobacter pylori Endosymbiont in Acanthamoeba-Positive Samples in Laboratory Conditions and in the Hospital Environments
- PMID: 39735838
- PMCID: PMC11671829
- DOI: 10.18502/ijpa.v19i4.17160
Molecular Diagnosis of Helicobacter pylori Endosymbiont in Acanthamoeba-Positive Samples in Laboratory Conditions and in the Hospital Environments
Abstract
Background: We aimed to identity Helicobacter pylori endosymbiont in Acanthamoeba-positive samples in natural and laboratory conditions.
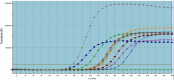
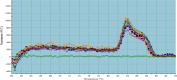
Methods: Overall, 134 samples were collected from hospital environments. Microscopic and PCR test were used for detection of Acanthamoeba and H. pylori. The real-time PCR method was used to check the active presence of H. pylori within Acanthamoeba under natural conditions from hospital samples and in co-culture laboratory conditions.
Results: The rate of contamination of hospital samples with Acanthamoeba was 44.7%. Out of 42 Acanthamoeba PCR-positive samples, 13 isolates (31%) were positive in terms of H. pylori endosymbiont according to sampling location. H. pylori is able to penetrate and enter the Acanthamoeba parasite.
Conclusion: H. pylori is able to contaminate Acanthamoeba in natural and laboratory conditions. The presence of pathogenic Acanthamoeba in various hospital environments and the hiding of Helicobacter as an endosymbiont inside it can pose a serious threat to the health of hospitalized patients.
Keywords: Acanthamoeba; Endosymbiont; Helicobacter pylori; Hospital.
© 2024 Mohammadi et al. Published by Tehran University of Medical Sciences.
Conflict of interest statement
Conflict of Interest There were no specific limitations that precluded the work, and the authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Detection of Acanthamoeba Harboring Campylobacter jejuni Endosymbionts in Hospital Environments of Markazi Province, Iran.J Parasitol Res. 2025 Feb 14;2025:6626888. doi: 10.1155/japr/6626888. eCollection 2025. J Parasitol Res. 2025. PMID: 39991014 Free PMC article.
-
Detection of viable Helicobacter pylori inside free-living amoebae in wastewater and drinking water samples from Eastern Spain.Environ Microbiol. 2017 Oct;19(10):4103-4112. doi: 10.1111/1462-2920.13856. Epub 2017 Aug 15. Environ Microbiol. 2017. PMID: 28707344
-
DVC-FISH and PMA-qPCR techniques to assess the survival of Helicobacter pylori inside Acanthamoeba castellanii.Res Microbiol. 2016 Jan;167(1):29-34. doi: 10.1016/j.resmic.2015.08.002. Epub 2015 Sep 1. Res Microbiol. 2016. PMID: 26342651
-
Acanthamoeba castellanii supports extracellular survival of Helicobacter pylori in co-culture.J Infect Chemother. 2020 Sep;26(9):946-954. doi: 10.1016/j.jiac.2020.04.016. Epub 2020 May 21. J Infect Chemother. 2020. PMID: 32448734
-
Prevalence of Helicobacter and Acanthamoeba in natural environment.Lett Appl Microbiol. 2009 Apr;48(4):465-71. doi: 10.1111/j.1472-765X.2008.02550.x. Epub 2009 Feb 2. Lett Appl Microbiol. 2009. PMID: 19187492
References
-
- Landell M, Salton J, Caumo K, Broetto L, Rott M. Isolation and genotyping of free-living environmental isolates of Acanthamoeba spp. from bromeliads in Southern Brazil. Exp Parasitol. 2013;134(3):290–4. - PubMed
-
- Corsaro D, Walochnik J, Köhsler M, Rott MB. Aanthamoeba misidentification and multiple labels: redefining genotypes T16, T19, and T 20and proposal for Acanthamoeba micheli sp. nov. (genotype T19). Parasitol Res. 2015; 114(7):2481–90. - PubMed
-
- Haniloo A, Pezeshki A, Mahmmodzadeh A, Kadkhodamohammadi E. Genotyping of Acanthamoeba spp. from water sources from Northwestern Iran. Acta Parasitol. 2017; 62(4): 790–795. - PubMed
-
- Steinert M. Pathogen–host interactions in Dictyostelium, Legionella, Mycobacterium and other pathogens. Semin Cell Dev Biol. 2011;22(1):70–6. - PubMed
LinkOut - more resources
Full Text Sources