Cholinesterase Inhibitory Activity of Paeoniflorin: Molecular Dynamics Simulation, and In Vitro Mechanistic Investigation
- PMID: 39735856
- PMCID: PMC11671635
- DOI: 10.1155/bri/9192496
Cholinesterase Inhibitory Activity of Paeoniflorin: Molecular Dynamics Simulation, and In Vitro Mechanistic Investigation
Abstract
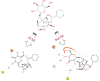
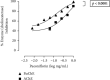
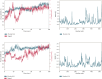
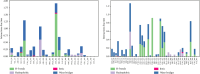
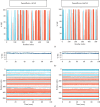
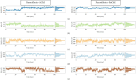
Alzheimer's disease (AD), a neurological disorder, is one of the major reasons for memory loss in the world. AD is characterized by a sequela of cognitive and functional decline caused by brain cell degeneration. Paeoniflorin is a monoterpenoid glycoside found in plants of the Paeoniaceae family, which are known for their medicinal properties including dementia. In this project, we report actions of paeoniflorin on the two related cholinesterases (ChE): acetylChE (AChE) and butyrylChE (BuChE). Paeoniflorin, in a dose-dependent (maximum inhibition at 1 mg/mL) manner, inhibited both AChE (0.06-1 mg/mL) and BuChE (0.007-1 mg/mL) enzymes with maximum inhibition of AChE enzyme at 90.3 ± 1.4%, while 99.4 ± 0.3% for BuChE enzyme. The EC50 value for the inhibitory effect of the compound against AChE was 0.52 mg/mL (0.18-1.52), while against BuChE was 0.13 mg/mL (0.08-0.21). The observed ani-ChE action was like an effect also mediated by the known ChE blocker physostigmine. Molecular interactions between paeoniflorin and both ChE enzymes were additionally sought via molecular docking and molecular dynamics simulations for 100 ns, that showed paeoniflorin interacted with the active-site gorge of AChE and BuChE via hydrogen bonds and water bridging with the many amino acids of the AChE and BuChE enzymes. This study presents the ChE inhibitory potential of paeoniflorin against both AChE and BuChE enzymes. With this kind of inhibitory activity, the chemical can potentially increase ACh levels and may have use in the treatment of dementia of AD.
Keywords: Alzheimer's disease; Paeoniaceae; acetylcholinesterase; butyrylcholinesterase; docking; enzyme inhibition; memory.
Copyright © 2024 Mohnad Abdalla et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- World Health Organization. Dementia . 2022 https://www.who.int/news-room/fact-sheets/detail/dementia .
-
- Soria Lopez J. A., González H. M., Léger G. C. Alzheimer’s Disease. In: Dekosky S. T., Asthana S., editors. Handbook of Clinical Neurology . Cambridge MA: Elsevier; 2019. pp. 231–255. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

