Prognostic Value of KRAS/ TP53 Status for Overall Survival in First-Line Monoimmunotherapy and Chemoimmunotherapy Treated Patients With Nonsquamous NSCLC in the Netherlands: A Brief Report
- PMID: 39735888
- PMCID: PMC11671682
- DOI: 10.1016/j.jtocrr.2024.100745
Prognostic Value of KRAS/ TP53 Status for Overall Survival in First-Line Monoimmunotherapy and Chemoimmunotherapy Treated Patients With Nonsquamous NSCLC in the Netherlands: A Brief Report
Abstract
Introduction: Programmed death-ligand 1 (PD-L1) is the main predictive biomarker used to identify patients with NSCLC who are eligible for treatment with immune checkpoint inhibitors. Despite its utility, the predictive capacity of PD-L1 is limited, necessitating the exploration of supplementary predictive biomarkers. In this report, we describe the prognostic value of KRAS/TP53 mutation status for overall survival (OS) in patients with NSCLC treated with first-line immunotherapy or combined chemoimmunotherapy.
Methods: Clinical data of all patients diagnosed with metastatic nonsquamous NSCLC in the Netherlands between January 1 and December 31 of 2019 were retrieved from the Netherlands Cancer Registry and linked to pathology reports of the Dutch Nationwide Pathology Databank. A total of 694 patients with available KRAS and TP53 mutation status and treated with first-line pembrolizumab or chemoimmunotherapy were included, with a median follow-up time of 42.5 months. Patients with an EGFR or MET mutation or ALK, ROS1, or RET fusion were excluded from the analysis.
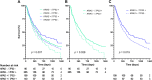
Results: Among patients treated with first-line pembrolizumab or chemoimmunotherapy, mutations in KRAS and TP53 occurred in 48.8% (n = 339) and 58.4% (n = 405), respectively. OS differed significantly between KRAS/TP53 mutational subgroups in patients treated with first-line pembrolizumab or chemoimmunotherapy (log-rank test, p = 0.007). Median OS of pembrolizumab or chemoimmunotherapy treated patients with mutated TP53 was longer in patients with KRAS-wildtype (485 versus 359 d, hazard ratio [HR] = 0.76, p = 0.028) or mutated KRAS (571 versus 447 d, HR = 0.73, p = 0.019). In a separate analysis of treatment subgroups, mutated TP53 was associated with longer median OS in chemoimmunotherapy treated KRAS-wildtype patients (468 versus 341 d, HR = 0.71, p = 0.029) but not in monoimmunotherapy treated patients with KRAS-wildtype (512 versus 371 d, HR = 0.91, p = 0.78). In multivariable Cox regression analysis including age, sex, clinical disease stage, and PD-L1 tumor proportion score, KRAS/TP53 mutation status was no longer associated with OS.
Conclusions: Among patients with metastatic NSCLC who are treated with pembrolizumab or chemoimmunotherapy, the presence of a pathogenic TP53 and KRAS mutation is associated with longer OS. Nevertheless, in multivariable Cox regression analysis including age, sex, clinical disease stage, and PD-L1 tumor proportion score, KRAS/TP53 mutation status was no longer associated with OS.
Keywords: Chemoimmunotherapy; Immunotherapy; KRAS; NSCLC; Real-world data; TP53.
© 2024 The Authors.
Conflict of interest statement
Dr. de Jager has received speaker’s fees from Roche and Janssen (Johnson&Johnson) (all paid to the institution). Dr. van Kempen has received grants or contracts from 10.13039/100002429Amgen, 10.13039/100004325AstraZeneca, 10.13039/100004326Bayer, Janssen-Cilag, Merck, Roche, and Servier (all payments to institution), has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Roche (all payments to institution), has received support for attending meetings or travel from Roche (payments to institution), has participated on a Data Safety Monitoring Board or Advisory Board for Janssen-Cilag, Merck, and Roche (all payments to institution), has a leadership or fiduciary role in Commission Personalized Medicine – Belgium (unpaid), has stock or stock options in Cyclomis (institutional/personal). Dr. Hiltermann has received grants or contracts from 10.13039/100004337Roche, AstraZeneca, and Bristol-Myers Squibb, and has leadership or fiduciary roles in CieBOM. Dr. Wekken has received grants or contracts from AstraZeneca, Boehringer Ingelheim, Pfizer, Roche, and Takeda, has received consulting fees from AstraZeneca, Janssen, Eli Lilly, Roche, and Takeda, has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Pfizer, and Roche, has a leadership or fiduciary role in oncology section NVALT, guideline committee NSCLC and CUP, dure geneesmiddelen committee NVALT and FMS. Dr. Schuuring has received grants or contracts from Abbott, Biocartis, AstraZeneca, Invitae/Archer, Bayer, Bio-Rad, Roche, Agena Bioscience, CC Diagnostics, Merck Sharp & Dohme/Merck, and SNN/EFRO (all (unrestricted) grants paid to UMCG institution), has received consulting fees from Merck Sharp & Dohme/Merck, AstraZeneca, Roche, Novartis, Bayer, Bristol-Myers Squibb, Eli Lilly, Amgen, Agena Bioscience, CC Diagnostics, and Janssen-Cilag (Johnson&Johnson) (all advisory board (incidental) and travel expenses (honoria/grant paid to UMCG institution)), and Astellas Pharma, GlaxoSmithKline, Sinnovisionlab, Sysmex, and Protyon (all advisory board (incidental) (honoria/grant paid to UMCG institution)), has received payments or honoraria for lectures, presentations, speakers bureaus, manuscript or educational events from Bio-Rad, Seracare, Roche, Biocartis, Eli Lilly, Agena Bioscience, Illumina, has received support for attending meeting or travel from Bio-Rad, Biocartis, Ageno Sciences, Illumina, Roche/Foundation Medicine, and QCMD, is board member of Dutch Society of Pathology (unpaid), European Society of Pathology (unpaid), European Liquid Biopsy Society (unpaid), is secretary/member of advisory committee for assessment of molecular diagnostics (cieBOD) (honoraria paid to UMCG institution), is member of the national guideline advisory committee (honoraria paid to UMCG institution). Dr. Willems has received grants or contracts from Roche, Bayer, Eli Lilly, Pfizer, AstraZeneca, Merck Sharp & Dohme, Amgen, and Novartis (unrestricted research grants), and has a leadership or fiduciary role in the strategic advisory board of Roche. Dr. Garcia declares no conflict of interest. The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL scientific staff for scientific advise.
Figures
References
-
- Bischoff P., Reck M., Overbeck T., et al. Outcome of first-line treatment with pembrolizumab according to KRAS/TP53 mutational status for nonsquamous programmed death-ligand 1-high (>/=50%) NSCLC in the German national network genomic medicine lung cancer. J Thorac Oncol. 2024;19:803–817. - PubMed
-
- Tønnesen E.M.T., Stougaard M., Meldgaard P., Lade-Keller J. Prognostic value of KRAS mutations, TP53 mutations and PD-L1 expression among lung adenocarcinomas treated with immunotherapy. J Clin Pathol. 2023;77:54–60. - PubMed
-
- Garcia B.N.C., van Kempen L.C., Kuijpers C.C.H.J., Schuuring E., Willems S.M., van der Wekken A.J. Prevalence of KRAS p.(G12C) in stage IV NSCLC patients in the Netherlands; a nation-wide retrospective cohort study. Lung Cancer. 2022;167:1–7. - PubMed
-
- Gijtenbeek R.G.P., Damhuis R.A.M., van der Wekken A.J., Hendriks L.E.L., Groen H.J.M., van Geffen W.H. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in the Netherlands: a retrospective, nationwide registry study. Lancet Reg Health Eur. 2023;27 - PMC - PubMed
-
- de Jager V.D., Cajiao Garcia B.N., Kuijpers C.C.H.J., et al. Regional differences in predictive biomarker testing rates for patients with metastatic NSCLC in the Netherlands. Eur J Cancer. 2024;205 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

