Transition-Metal-Free Thioboration of Terminal Alkynes
- PMID: 39735905
- PMCID: PMC11672135
- DOI: 10.1021/jacsau.4c00907
Transition-Metal-Free Thioboration of Terminal Alkynes
Abstract
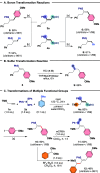
We present a new type of elementoboration reaction, the thioboration of terminal alkynes. This method enables highly controllable regio-/stereo-/chemoselective cis- and trans-thioboration on demand, affording synthetically versatile and densely functionalized vinyl boron/vinyl sulfide derivatives in a straightforward manner without the need for a transition-metal catalyst.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
In Situ Generation of Silylzinc by Si-B Bond Activation Enabling Silylzincation and Silaboration of Terminal Alkynes.Angew Chem Int Ed Engl. 2018 Jul 2;57(27):8053-8057. doi: 10.1002/anie.201802887. Epub 2018 May 30. Angew Chem Int Ed Engl. 2018. PMID: 29696748
-
Catalyst-Free Formal Thioboration to Synthesize Borylated Benzothiophenes and Dihydrothiophenes.Angew Chem Int Ed Engl. 2016 Nov 7;55(46):14286-14290. doi: 10.1002/anie.201608090. Epub 2016 Oct 13. Angew Chem Int Ed Engl. 2016. PMID: 27735114 Free PMC article.
-
Stereoselective Access to Highly Substituted Vinyl Ethers via trans-Difunctionalization of Alkynes with Alcohols and Iodine(III) Electrophile.J Am Chem Soc. 2020 May 13;142(19):8619-8624. doi: 10.1021/jacs.0c04140. Epub 2020 May 5. J Am Chem Soc. 2020. PMID: 32362119
-
Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes.Chem Soc Rev. 2014 Mar 7;43(5):1575-600. doi: 10.1039/c3cs60369e. Epub 2013 Dec 16. Chem Soc Rev. 2014. PMID: 24336638 Review.
-
Recent advances in the ruthenium-catalyzed hydroarylation of alkynes with aromatics: synthesis of trisubstituted alkenes.Org Biomol Chem. 2015 Nov 14;13(42):10420-36. doi: 10.1039/c5ob01472g. Epub 2015 Sep 18. Org Biomol Chem. 2015. PMID: 26383714 Review.
References
-
- Negishi E. Magical Power of Transition Metals: Past, Present, and Future. Angew. Chem., Int. Ed. 2011, 50, 6738–6764. 10.1002/anie.201101380. - DOI - PubMed
- Yoshida H. Borylation of Alkynes under Base/Coinage Metal Catalysis: Some Recent Developments. ACS Catal. 2016, 6, 1799–1811. 10.1021/acscatal.5b02973. - DOI
- Ansell M. B.; Navarro O.; Spencer J. Transition metal catalyzed element–element’ additions to alkynes. Coord. Chem. Rev. 2017, 336, 54–77. 10.1016/j.ccr.2017.01.003. - DOI
- Whyte A.; Torelli A.; Mirabi B.; Zhang A.; Lautens M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. 10.1021/acscatal.0c02758. - DOI
- Altarejos J.; Valero A.; Manzano R.; Carreras J. Synthesis of Tri- and Tetrasubstituted Alkenyl Boronates from Alkynes. Eur. J. Org Chem. 2022, 2022, e20220052110.1002/ejoc.202200521. - DOI
-
-
Initial reports (B–H):
- Brown H. C.; Rao B. C. S. A New Technique for the Conversion of Olefins into Organoboranes and Related Alcohols. J. Am. Chem. Soc. 1956, 78, 5694–5695. 10.1021/ja01602a063. - DOI
- Brown H. C.; Chen J. Hydroboration. 57. Hydroboration with 9-borabicyclo[3.3.1]nonane of alkenes containing representative functional groups. J. Org. Chem. 1981, 46, 3978–3988. 10.1021/jo00333a009. - DOI
-
-
-
Recent representative reviews (B–H):
- Rej S.; Das A.; Panda T. K. Overview of Regioselective and Stereoselective Catalytic Hydroboration of Alkynes. Adv. Synth. Catal. 2021, 363, 4818–4840. 10.1002/adsc.202100950. - DOI
- Magre M.; Szewczyk M.; Rueping M. s-Block Metal Catalysts for the Hydroboration of Unsaturated Bonds. Chem. Rev. 2022, 122, 8261–8312. 10.1021/acs.chemrev.1c00641. - DOI - PMC - PubMed
-
-
-
Early reports (B–Halogen):
- Lappert M. F.; Prokai B. Chloroboration and Allied Reactions of Unsaturated Compounds II. Haloboration and Phenylboration of Acetylenes; and the Preparation of Some Alkynylboranes. J. Organomet. Chem. 1964, 1, 384–400. 10.1016/S0022-328X(00)80030-3. - DOI
- Joy F.; Lappert M. F.; Prokai B. Chloroboration and Allied Reactions of Unsaturated Compounds: V. Haloboration and Phenyl-boration of Olefins; and the Preparation of Hexaphenyl-1,4-Diboracyclohexa-2,5-Diene. J. Organomet. Chem. 1966, 5, 506–519. 10.1016/S0022-328X(00)85153-0. - DOI
-
LinkOut - more resources
Full Text Sources
