Concatenating Microbial, Enzymatic, and Organometallic Catalysis for Integrated Conversion of Renewable Carbon Sources
- PMID: 39735920
- PMCID: PMC11672146
- DOI: 10.1021/jacsau.4c00511
Concatenating Microbial, Enzymatic, and Organometallic Catalysis for Integrated Conversion of Renewable Carbon Sources
Abstract
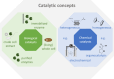
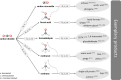
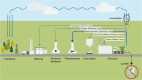
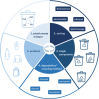
The chemical industry can now seize the opportunity to improve the sustainability of its processes by replacing fossil carbon sources with renewable alternatives such as CO2, biomass, and plastics, thereby thinking ahead and having a look into the future. For their conversion to intermediate and final products, different types of catalysts-microbial, enzymatic, and organometallic-can be applied. The first part of this review shows how these catalysts can work separately in parallel, each route with unique requirements and advantages. While the different types of catalysts are often seen as competitive approaches, an increasing number of examples highlight, how combinations and concatenations of catalysts of the complete spectrum can open new roads to new products. Therefore, the second part focuses on the different catalysts either in one-step, one-pot transformations or in reaction cascades. In the former, the reaction conditions must be conflated but purification steps are minimized. In the latter, each catalyst can work under optimal conditions and the "hand-over points" should be chosen according to defined criteria like minimal energy usage during separation procedures. The examples are discussed in the context of the contributions of catalysis to the envisaged (bio)economy.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Jiménez-González C.; Poechlauer P.; Broxterman Q. B.; Yang B.-S.; Ende D. am; Baird J.; Bertsch C.; Hannah R. E.; Dell’Orco P.; Noorman H.; Yee S.; Reintjens R.; Wells A.; Massonneau V.; Manley J. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Process Res. Dev. 2011, 15 (4), 900–911. 10.1021/op100327d. - DOI
Publication types
LinkOut - more resources
Full Text Sources
