Unlocking Mesoscopic Disorder in Graphitic Carbon with Spectroelectrochemistry
- PMID: 39736149
- PMCID: PMC11848973
- DOI: 10.1002/anie.202420680
Unlocking Mesoscopic Disorder in Graphitic Carbon with Spectroelectrochemistry
Abstract
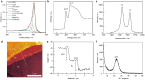
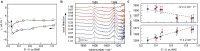
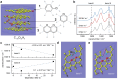
Intrinsic structural and oxidic defects activate graphitic carbon electrodes towards electrochemical reactions underpinning energy conversion and storage technologies. Yet, these defects can also disrupt the long-range and periodic arrangement of carbon atoms, thus, the characterization of graphitic carbon electrodes necessitates in-situ atomistic differentiation of graphitic regions from mesoscopic bulk disorder. Here, we leverage the combined techniques of in-situ attenuated total reflectance infrared spectroscopy and first-principles calculations to reveal that graphitic carbon electrodes exhibit electric-field dependent infrared activity that is sensitive to the bulk mesoscopic intrinsic disorder. With this platform, we identify graphitic regions from amorphous domains by discovering that they demonstrate opposing electric-field-dependent infrared activity under electrochemical conditions. Our work provides a roadmap for identifying mesoscopic disorder in bulk carbon materials under potential bias.
Keywords: Disordered graphitic carbon electrodes; first-principles calculations; in-situ infrared spectroscopy.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- McCreery R. L., Chem. Rev. 2008, 108, 2646–2687. - PubMed
-
- Zhu J., Mu S., Adv. Funct. Mater. 2020, 30, 2001097.
-
- Jia Y., Yao X., Acc. Chem. Res. 2023, 56, 948–958. - PubMed
-
- Jia Y., Zhang L., Zhuang L., Liu H., Yan X., Wang X., Liu J., Wang J., Zheng Y., Xiao Z., Taran E., Chen J., Yang D., Zhu Z., Wang S., Dai L., Yao X., Nat. Catal. 2019, 2, 688–695.
-
- Wang X., Jia Y., Mao X., Zhang L., Liu D., Song L., Yan X., Chen J., Yang D., Zhou J., Wang K., Du A., Yao X., Chem. 2020, 6, 2009–2023.
Grants and funding
LinkOut - more resources
Full Text Sources

