Enhanced dynorphin expression and secretion in pancreatic beta-cells under hyperglycemic conditions
- PMID: 39736444
- PMCID: PMC11846442
- DOI: 10.1016/j.molmet.2024.102088
Enhanced dynorphin expression and secretion in pancreatic beta-cells under hyperglycemic conditions
Abstract
Objective: Dynorphin, an endogenous opioid peptide predominantly expressed in the central nervous system and involved in stress response, pain, and addiction, has intrigued researchers due to its expression in pancreatic β-cells. In this study, we aimed to characterize dynorphin expression in mouse and human islets and explore the mechanisms regulating its expression.
Methods: We used primary mouse and human islets with unbiased published datasets to examine how glucose and other nutrients regulate dynorphin expression and secretion in islets.
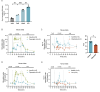
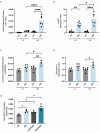
Results: The prodynorphin gene is significantly upregulated in β-cells under hyperglycemic conditions. In vitro studies revealed that increased glucose concentrations correlate with increased dynorphin expression, indicating a critical interplay involving Ca2+, CamKII, and CREB pathways in β-cells. Perifusion studies allowed us to measure the dynamic secretion of dynorphin in response to glucose from mouse and human islets for the first time. Furthermore, we confirmed that increased dynorphin content within the β-cells directly correlates with enhanced dynorphin secretion. Finally, our findings demonstrate a synergistic effect of palmitate in conjunction with high glucose, further amplifying dynorphin levels and secretion in pancreatic islets.
Conclusions: This study demonstrates that the opioid peptide prodynorphin is expressed in mouse and human β-cells. Prodynorphin levels are regulated in parallel with insulin in response to glucose, palmitate, and amino acids. Our findings elucidate the signaling pathways involved, with CamKII playing a key role in regulating prodynorphin levels in β-cells. Finally, our findings are the first to demonstrate active dynorphin secretion from mouse and human islets in response to glucose.
Keywords: Beta-cell; CamKII; Dynorphin; Islet.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Manuel Blandino-Rosano reports financial support was provided by National Institute of Health (NIDDK). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Schinner S., Scherbaum W.A., Bornstein S.R., Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005;22(6):674–682. - PubMed
-
- Bhattacharya S., Dey D., Roy S.S. Molecular mechanism of insulin resistance. J Biosci. 2007;32(2):405–413. - PubMed
-
- Prevention, C.f.D.C.a., About underlying cause of death 1999–2015. CDC WONDER database.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

