Risk factors for methotrexate treatment failure in tubal ectopic pregnancy: a retrospective cohort study
- PMID: 39736575
- PMCID: PMC11686986
- DOI: 10.1186/s12884-024-07122-6
Risk factors for methotrexate treatment failure in tubal ectopic pregnancy: a retrospective cohort study
Abstract
Background: Ectopic pregnancy (EP) accounts for approximately 2% of all pregnancies, with tubal ectopic pregnancies (TEPs) being the most common type. Methotrexate (MTX) is a noninvasive and effective medical management option for TEP, but failure rates range from 10 to 36%, posing challenges in clinical practice. Identifying risk factors for MTX treatment failure is crucial to improve patient outcomes and guide clinical decision-making. This study aimed to determine the risk factors associated with MTX failure in TEP patients and support personalized treatment strategies.
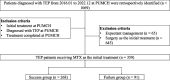
Methods: This retrospective study included female patients who were diagnosed with TEP at Peking Union Medical College Hospital (PUMCH) between January 2016 and December 2022. Patients received MTX treatment initially, with dosing intervals and protocols varying according to clinical practice. MTX treatment failure was defined as the need for surgery after MTX administration. The study included two groups: patients who failed MTX treatment (n = 91) and those who succeeded in treatment (n = 268). Univariate and multivariate logistic regression analyses were performed to identify significant predictors of MTX treatment failure. A nomogram was developed to visualize the predictive factors.
Results: A total of 359 patients were included, with 268 (74.7%) succeeding with MTX and 91 (25.3%) required surgery. Specifically, 282 patients (78.6%) received 1-dose MTX, whereas 77 (21.4%) received 2-dose MTX. Univariate analysis revealed that gravidity, previous EP, gestational age, pretreatment β-human chorionic gonadotropin (β-hCG) level, number of MTX treatments, and presence of a visible yolk sac in ultrasound were significant predictors (all P < 0.05). Multivariate analysis confirmed that higher gravidity (odds ratio (OR) = 1.2487, 95% confidence interval (CI): 1.0103 - 1.5433, P = 0.040) and elevated pretreatment β-hCG levels (OR = 1.0006, 95% CI: 1.0004 - 1.0008, P < 0.001) were independent risk factors. Number of MTX treatments was a significant protective factor (OR = 0.4409, 95% CI: 0.2153 - 0.9025, P = 0.025). The nomogram incorporating these six risk factors was developed.
Conclusion: Higher gravidity and elevated β-hCG levels were significant predictors of MTX failure, while more MTX doses provided a protective effect. These findings underscore the importance of personalized MTX treatment strategies to improve outcomes in TEP. However, the limitations of this study, including its retrospective and single-center design, suggest that further prospective multicenter studies are needed to validate these results.
Trial registration: The trial is registered at http://www.chictr.org.cn . [registration number: ChiCTR2400081314; registration date: 2024-02-28 (prospectively registered)].
Keywords: Ectopic pregnancy; Methotrexate; Tubal pregnancy; β-human chorionic gonadotropin.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the Institutional Review Board of the Peking Union Medical College Hospital (PUMCH) (No. I—24PJ0349). Informed consent was obtained from all participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
The predictors of successful methotrexate treatment of tubal ectopic pregnancy.J Obstet Gynaecol. 2024 Dec;44(1):2361456. doi: 10.1080/01443615.2024.2361456. Epub 2024 Jun 12. J Obstet Gynaecol. 2024. PMID: 38864434
-
Nomogram to predict methotrexate treatment success in ectopic pregnancy.Int J Gynaecol Obstet. 2025 Feb;168(2):620-627. doi: 10.1002/ijgo.15927. Epub 2024 Sep 26. Int J Gynaecol Obstet. 2025. PMID: 39324483
-
Predictors of treatment failure of tubal pregnancy with single-dose methotrexate regimen - a systematic review and meta-analysis.J Obstet Gynaecol. 2025 Dec;45(1):2447997. doi: 10.1080/01443615.2024.2447997. Epub 2025 Jan 7. J Obstet Gynaecol. 2025. PMID: 39773144
-
Multidose Methotrexate Treatment of Ectopic Pregnancies with High initial β-Human Chorionic Gonadotropin: Can Success Be Predicted?Gynecol Obstet Invest. 2019;84(1):56-63. doi: 10.1159/000491083. Epub 2018 Aug 10. Gynecol Obstet Invest. 2019. PMID: 30099453
-
Interventions for non-tubal ectopic pregnancy.Cochrane Database Syst Rev. 2020 Jul 1;7(7):CD011174. doi: 10.1002/14651858.CD011174.pub2. Cochrane Database Syst Rev. 2020. PMID: 32609376 Free PMC article.
Cited by
-
The application of super-resolution ultrasound radiomics models in predicting the failure of conservative treatment for ectopic pregnancy.Reprod Biol Endocrinol. 2025 Jul 17;23(1):102. doi: 10.1186/s12958-025-01437-5. Reprod Biol Endocrinol. 2025. PMID: 40676578 Free PMC article.
References
-
- American College of O, Gynecologists' Committee on Practice B-G. ACOG Practice Bulletin No. 193: Tubal Ectopic Pregnancy. Obstet Gynecol. 2018;131(3):e91-e103. - PubMed
-
- Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstet Gynecol. 2011;117(4):837–43. - PubMed
-
- National Institute for Health and Care Excellence: Guidelines. Ectopic pregnancy and miscarriage: diagnosis and initial management. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2023.; 2023. - PubMed
-
- Revzin MV, Pellerito JS, Moshiri M, Katz DS, Nezami N, Kennedy A. Use of methotrexate in gynecologic and obstetric practice: what the radiologist needs to know. Radiographics. 2021;41(6):1819–38. - PubMed
-
- Cai H, Mol BW, Li P, Liu X, Watrelot A, Shi J. Tubal factor infertility with prior ectopic pregnancy: a double whammy? A retrospective cohort study of 2,892 women. Fertil Steril. 2020;113(5):1032–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

