Distinct regulation of Tau Monomer and aggregate uptake and intracellular accumulation in human neurons
- PMID: 39736627
- PMCID: PMC11686972
- DOI: 10.1186/s13024-024-00786-w
Distinct regulation of Tau Monomer and aggregate uptake and intracellular accumulation in human neurons
Abstract
Background: The prion-like spreading of Tau pathology is the leading cause of disease progression in various tauopathies. A critical step in propagating pathologic Tau in the brain is the transport from the extracellular environment and accumulation inside naïve neurons. Current research indicates that human neurons internalize both the physiological extracellular Tau (eTau) monomers and the pathological eTau aggregates. However, similarities or differences in neuronal transport mechanisms between Tau species remain elusive.
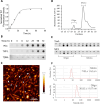
Method: Monomers, oligomers, and fibrils of recombinant 2N4R Tau were produced and characterized by biochemical and biophysical methods. A neuronal eTau uptake and accumulation assay was developed for human induced pluripotent stem cell-derived neurons (iPSCNs) and Lund human mesencephalic cells (LUHMES)-derived neurons. Mechanisms of uptake and cellular accumulation of eTau species were studied by using small molecule inhibitors of endocytic mechanisms and siRNAs targeting Tau uptake mediators.
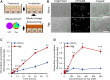
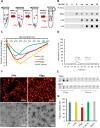
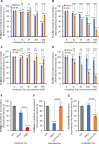
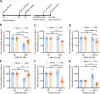
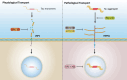
Results: Extracellular Tau aggregates accumulated more than monomers in human neurons, mainly due to the higher efficiency of small fibrillar and soluble oligomeric aggregates in intraneuronal accumulation. A competition assay revealed a distinction in the neuronal accumulation between physiological eTau Monomers and pathology-relevant aggregates, suggesting differential transport mechanisms. Blocking heparan sulfate proteoglycans (HSPGs) with heparin only inhibited the accumulation of eTau aggregates, whereas monomers' uptake remained unaltered. At the molecular level, the downregulation of genes involved in HSPG synthesis exclusively blocked neuronal accumulation of eTau aggregates but not monomers, suggesting its role in the transport of pathologic Tau. Moreover, the knockdown of LRP1, as a receptor of Tau, mainly reduced the accumulation of monomeric form, confirming its involvement in Tau's physiological transport.
Conclusion: These data propose that despite the similarity in the cellular mechanism, the uptake and accumulation of eTau Monomers and aggregates in human neurons are regulated by different molecular mediators. Thus, they address the possibility of targeting the pathological spreading of Tau aggregates without disturbing the probable physiological or non-pathogenic transport of Tau Monomers.
Keywords: Cell-to-cell spreading; Extracellular Tau; HSPGs; LRP1; Neurodegeneration; Uptake; VPS35.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The experiments were approved by the Ethics Committee of the Technical University of Munich (21/19 S-AS). Consent for publication: N/A. Competing interests: The authors declare that they have no competing interests to disclose.
Figures


References
-
- Rosler TW, Tayaranian Marvian A, Brendel M, Nykanen NP, Hollerhage M, Schwarz SC, et al. Four-repeat tauopathies. Prog Neurobiol. 2019;180:101644. - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261(13):6084–9. - PubMed
-
- Goedert M, Falcon B, Clavaguera F, Tolnay M. Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr Neurol Neurosci Rep. 2014;14(11):495. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

