Migrated intra-uterine device to infra-umbilical skin: a rare case report
- PMID: 39736668
- PMCID: PMC11684260
- DOI: 10.1186/s12905-024-03522-0
Migrated intra-uterine device to infra-umbilical skin: a rare case report
Abstract
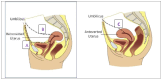
Introduction: IUDs are effective, reversible and safe methods of contraception. The mechanism of action of IUDs as a group is inducing endometrial atrophy, apoptosis, altering tubal motility; preventing sperm permeability, fertilization, and implantation. Complications of IUD include menstrual disturbance, pelvic pain, and increased risk of ectopic pregnancy with contraceptive failure, device expulsion, uterine perforation or transmural migration with misplacement of the device. Pregnancies and IUD migration are uncommon complications, occurring in 1 to 2 from 1,000 users. The clinical presentation of migrated IUDs depends on the final anatomic location at diagnosis. Migration can be asymptomatic and incidentally found while imaging for any other diagnosis or may have various acute clinical presentations.
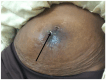
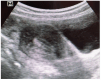
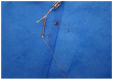
Case presentation: We present the case of a 38-years-old Ethiopian woman with unusual migration of IUD to the infra-umbilical skin. She had infra-umbilical skin discoloration associated with intermittent itching for two months duration. On physical examination, there was diffuse violaceous patch over the infra-umbilical skin measuring 7 centimeters on its longest dimension and visible foreign body (IUD) with tiny sinus tract formation. Visible stem of copper-containing IUD was grasped by ring forceps and removed with gentle traction without complication during or following extraction. Removal of misplaced IUD with completely non-invasive manner makes this case special in addition to the unique site of migration to the infra-umbilical skin.
Conclusion and recommendation: To our knowledge, this is what likely to be the first reported case of IUD migration to the infra-umbilical skin which bestows a new finding to the existing literatures. Despite rare occurrence, possible IUD complications should be included in the informed consent process before insertion. Self-examination of the strings and vigilant evaluation at regular checkup is recommended for early detection of migrated IUDs.
Keywords: Case report; Infra-umbilical skin; Intrauterine device; Migration; Violaceous Patch.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and patient consent: This case report has been submitted to the school of medicine at University of Gondar College of medicine and health sciences for ethical board review and was approved as morally sound report. A written informed consent was obtained from the patient for publication of the case report and accompanying images. A copy of patient’s written consent is available for review for the editor-in-chief of this journal. Conflict of interest: None Declared.
Figures
Similar articles
-
Intrauterine devices: an effective alternative to oral hormonal contraception.Prescrire Int. 2009 Jun;18(101):125-30. Prescrire Int. 2009. PMID: 19637436
-
Uterine perforation by copper intrauterine device.Eur J Obstet Gynecol Reprod Biol. 1984 Jun;17(4):257-61. doi: 10.1016/0028-2243(84)90068-6. Eur J Obstet Gynecol Reprod Biol. 1984. PMID: 6378687
-
Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes.J Minim Invasive Gynecol. 2014 Jul-Aug;21(4):596-601. doi: 10.1016/j.jmig.2013.12.123. Epub 2014 Jan 21. J Minim Invasive Gynecol. 2014. PMID: 24462588 Free PMC article.
-
The pathology of intra-uterine contraceptive devices.Curr Top Pathol. 1994;86:307-30. doi: 10.1007/978-3-642-76846-0_8. Curr Top Pathol. 1994. PMID: 8162713 Review.
-
Progestin intrauterine devices versus copper intrauterine devices for emergency contraception.Cochrane Database Syst Rev. 2023 Feb 27;2(2):CD013744. doi: 10.1002/14651858.CD013744.pub2. Cochrane Database Syst Rev. 2023. PMID: 36847591 Free PMC article.
References
-
- Lanzola EL, Ketvertis K. Intrauterine device. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Kari Ketvertis declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024. StatPearls Publishing LLC.; 2024.
-
- Committee Opinion 672. Clinical challenges of Long-Acting Reversible Contraceptive methods. Obstet Gynecol. 2016;128(3):e69–77. - PubMed
-
- Grandi G, Farulla A, Sileo FG, Facchinetti F. Levonorgestrel-releasing intra-uterine systems as female contraceptives. Expert Opin Pharmacother. 2018;19(7):677–86. - PubMed
-
- Stephen Searle E. The intrauterine device and the intrauterine system. Best Pract Res Clin Obstet Gynecol. 2014;28(6):807–24. - PubMed
-
- Elahi N, Koukab H. Diagnosis and management of lost intrauterine contraceptive device. JPMA J Pakistan Med Association. 2002;52(1):18–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

