Oral health risks in adults who use electronic nicotine delivery systems and oral nicotine pouches: a critical review of the literature and qualitative synthesis of the available evidence
- PMID: 39736680
- PMCID: PMC11687081
- DOI: 10.1186/s12954-024-01147-y
Oral health risks in adults who use electronic nicotine delivery systems and oral nicotine pouches: a critical review of the literature and qualitative synthesis of the available evidence
Abstract
Background: Use of combustible cigarettes (CCs) and smokeless oral tobacco products are well documented risk factors for a variety of oral diseases. However, the potential oral health risks of using recently introduced (since about 2000) non-combustible tobacco/nicotine products (NCPs: electronic cigarettes (ECs), heated tobacco products (HTPs) and oral nicotine pouches (ONPs), remain poorly established.
Methods: This review evaluates published human studies on detrimental oral health effects in people who use NCPs compared to those smoking cigarettes and those not using any tobacco/nicotine product (NU). We identified 52 studies, predominantly focusing on adults who used electronic cigarettes as an NCP. The studies exhibited significant heterogeneity regarding design, populations, endpoints and quality. Reported outcomes, based on both single and grouped endpoints were qualitatively evaluated by comparing people who use NCPs with NU and with people smoking CCs. Significant increases (indicating a worsening in oral health), significant decreases (indicating a lower level of detrimental effects) and no significant difference between groups were assigned scores of + 1, -1 and 0, respectively. Scores from studies belonging to the same single or grouped endpoints were averaged to a summary score ranging from - 1 to + 1.
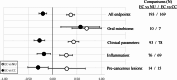
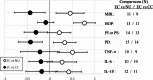
Results: The qualitative meta-analysis revealed that comparisons of EC versus NU groups yielded mean scores of 0.29 for pre-cancerous lesions (N = 14 observations), 0.27 for inflammatory processes (N = 83), 0.43 for oral clinical parameters (N = 93) and 0.70 for shifts in the oral microbiome (N = 10). The corresponding values for the EC versus CC group comparisons amounted to -0.33 (N = 15), -0.14 (N = 76), -0.27 (N = 78) and 0.57 (N = 7). Most studies had significant limitations regarding group sizes, duration of NCP use (mostly only a few years) and validity of self-reported exclusive NCP use. Notably, the implications of dual use (EC + CC) and prior CC use were often not adequately considered.
Conclusions: The evaluated studies suggest that use of ECs is associated with relatively fewer detrimental oral health effects compared to smoking, yet oral health status remains poorer compared to not using any tobacco/nicotine products. These results have to be interpreted with caution due to a number of limitations and uncertainties in the underlying studies, particularly the potential biases and confounding factors inherent in cross-sectional study designs.
Keywords: Combustible cigarettes; Electronic cigarettes; Heated tobacco products; Oral health; Oral nicotine pouches.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Tobacco Advisory Group of the Royal. College of Physicians (RCP), editor nicotine without smoke—tobacco harm reduction. Royal College of Physicians; 2016.
-
- US Department of Health and Human Services. The Healh Consequences of Using Smokeless Tobacco. A Report of the Surgeon General. 1986;http://resource.nlm.nih.gov/101584932X65
-
- International Agency for Research on Cancer (IARC). Tobacco smoke and involuntary smoking. Lyon, France: IARC Press; 2004 2004.
-
- Asthana S, Labani S, Kailash U, Sinha DN, Mehrotra R. Association of Smokeless Tobacco Use and oral Cancer: a systematic global review and Meta-analysis. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco; 2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

