Disrupting USP39 deubiquitinase function impairs the survival and migration of multiple myeloma cells through ZEB1 degradation
- PMID: 39736693
- PMCID: PMC11686864
- DOI: 10.1186/s13046-024-03241-2
Disrupting USP39 deubiquitinase function impairs the survival and migration of multiple myeloma cells through ZEB1 degradation
Abstract
Background: Multiple Myeloma (MM) is the second most common hematological malignancy, characterized by the accumulation of monoclonal plasmocytes in the bone marrow. Despite advancements with proteasome inhibitors, immunomodulatory agents, and CD38-targeting antibodies, MM remains largely incurable due to resistant clones and frequent relapses. The success of the proteasome inhibitor bortezomib (BTZ) in MM treatment highlights the critical role of the ubiquitin-proteasome system (UPS) in this disease. Deubiquitinases (DUBs), which regulate protein stability, interactions, and localization by removing ubiquitin modifications, have emerged as promising therapeutic targets in various cancers, including MM.
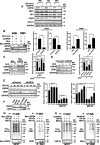
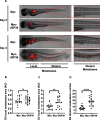
Methods: Through a comprehensive loss-of-function screen, we identified USP39 as a critical survival factor for MM cells. Gene Set Enrichment Analysis (GSEA) was employed to correlate USP39 mRNA levels with clinical outcomes in MM patients. USP39 protein expression was evaluated via immunohistochemistry (IHC) on bone marrow samples from MM patients and healthy controls. The impact of USP39 knockdown via SiRNA was assessed through in vitro assays measuring cellular metabolism, clonogenic capacity, cell cycle progression, apoptosis, and sensitivity to BTZ. Co-immunoprecipitation and deubiquitination assays were conducted to elucidate the interaction and regulation of ZEB1 by USP39. Finally, in vitro and in vivo zebrafish experiments were used to characterize the biological consequences of ZEB1 regulation by USP39.
Results: Our study found that elevated USP39 mRNA levels are directly associated with shorter survival in MM patients. USP39 protein expression is significantly higher in MM patient plasmocytes compared to healthy individuals. USP39 knockdown inhibits clonogenic capacity, induces cell cycle arrest, triggers apoptosis, and overcomes BTZ resistance. Gain-of-function assays revealed that USP39 stabilizes the transcription factor ZEB1, enhancing the proliferation and the trans-migratory potential of MM cells.
Conclusions: Our findings highlight the critical role of the deubiquitinase USP39, suggesting that the USP39/ZEB1 axis could serve as a potential diagnostic marker and therapeutic target in MM.
Keywords: Deubiquitinase; Migration; Multiple myeloma; USP39; ZEB1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All Zebrafish experiments were approved by the IRCAN Experimental Animal Ethical Committee. Consent for publication: All authors consent to publication. Competing interests: The authors have declared that no competing interests exist.
Figures






References
-
- Nandi D, Tahiliani P, Kumar A, Chandu D. The ubiquitin-proteasome system. J Biosci. 2006;31(1):137–55. 10.1007/BF02705243. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

