Sex differences in the relationships between 24-h rest-activity patterns and plasma markers of Alzheimer's disease pathology
- PMID: 39736697
- PMCID: PMC11684129
- DOI: 10.1186/s13195-024-01653-y
Sex differences in the relationships between 24-h rest-activity patterns and plasma markers of Alzheimer's disease pathology
Abstract
Background: Although separate lines of research indicated a moderating role of sex in both sleep-wake disruption and in the interindividual vulnerability to Alzheimer's disease (AD)-related processes, the quantification of sex differences in the interplay between sleep-wake dysregulation and AD pathology remains critically overlooked. Here, we examined sex-specific associations between circadian rest-activity patterns and AD-related pathophysiological processes across the adult lifespan.
Methods: Ninety-two cognitively unimpaired adults (mean age = 59.85 ± 13.77 years, range = 30-85, 47 females) underwent 10 days of actigraphic recordings, and blood drawing. Standard non-parametric indices of 24-h rest-activity rhythm fragmentation (intradaily variability, IV) and stability (interdaily stability, IS) were extracted from actigraphy data using the GGIR package. Plasma concentrations of neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), amyloid-β42/40 (Aβ42/40), total tau, and tau phosphorylated at threonine 181 (p-tau181) or threonine 231 (p-tau231) were measured using Single molecule array technology. Multiple linear regression models were adjusted for age, sex, education, body mass index, and actigraphic recording duration.
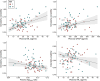
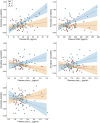
Results: Higher IV, indicating worse 24-h rest-activity rhythm fragmentation, was associated with elevated levels of plasma NfL (t(85) = 4.26, P < 0.0001), GFAP (t(85) = 2.49, P = 0.01), and at trend level with lower Aβ42/40 ratio values (t(85) = -1.95, P = 0.054). Lower IS, reflecting less day-to-day stability in the 24-h rest-activity rhythm, was linked to elevated levels of plasma NfL (t(85) = -2.24, P = 0.03), but not with the other plasma biomarkers. Importantly, interaction models demonstrated that male participants were driving the observed relationships between IV and plasma NfL (t(84) = 4.05, P < 0.001) or GFAP (t(84) = 3.60, P < 0.001), but also revealed a male vulnerability in models testing interactions with p-tau181 (IV: t(76) = 3.71, P < 0.001; IS: t(76) = -3.30, P = 0.001) and p-tau231 (IV: t(82) = 3.28, P = 0.002). Sensitivity analyses further showed that accounting for potential confounding factors such as APOE genotype, depression, and self-reported symptoms of possible sleep apnea did not modify the observed relationships.
Conclusions: These findings suggest that the association between disrupted circadian rest-activity patterns and AD pathophysiological processes may be more evident in cognitively unimpaired males. Our results contribute to the precision medicine approach, and they have clinical implications for improved early detection and selection of at-risk individuals to be enrolled in preventive interventions.
Keywords: 24-h rest-activity patterns; Actigraphy; Amyloid-beta; Glial fibrillary acidic protein; Interdaily stability; Intradaily variability; Neurofilament light chain; Plasma biomarkers; Sex differences; Tau.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the local medical ethics committee of the Faculty of Health, Medicine and Life Sciences at Maastricht University, Maastricht, the Netherlands (#METC183002), and was conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent prior to participation and received a monetary compensation. Consent for publication: Not applicable. Competing interests: N.J.A. has given lectures in symposia sponsored by Eli Lily, and is an associate editor at Alzheimer’s Research & Therapy. K.B. has served as a consultant at advisory boards or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work), and is member of the editorial board at Alzheimer’s Research & Therapy. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). H.I.L.J. is chair of the Neuromodulatory Subcortical Systems Professional Interest Area of ISTAART and advisory board member of ISTAART. All other authors report no conflict of interest.
Figures
References
-
- Van Egroo M, Narbutas J, Chylinski D, Villar González P, Maquet P, Salmon E, et al. Sleep–wake regulation and the hallmarks of the pathogenesis of Alzheimer’s disease. Sleep. 2019;42:1–13. - PubMed
-
- Rigat L, Ouk K, Kramer A, Priller J. Dysfunction of circadian and sleep rhythms in the early stages of Alzheimer’s disease. Acta Physiol. 2023;238:1–13. - PubMed
MeSH terms
Substances
Grants and funding
- ZEN-21-848495/Alzheimer's Association 2021 Zenith Award
- #ALFGBG-715986 and #ALFGBG-965240/Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement
- #2017-00915/Swedish Research Council
- P30 AG072980/AG/NIA NIH HHS/United States
- #FO2017-0243 and #ALZ2022-0006/Hjärnfonden
- JPND2019-466-236/European Union Joint Program for Neurodegenerative Disorders
- UKDRI-1003/UK Dementia Research Institute at UCL
- #FO2022-0270/Hjärnfonden
- 860197 (MIRIADE)/European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant
- #ALFGBG-71320/Swedish State Support for Clinical Research
- #ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- R01AG062559, R01AG06806, R01AG082006, and R21AG074220/NH/NIH HHS/United States
- #2023-00356; #2022-01018 and #2019-02397/Swedish Research Council
- A20211016F/BrightFocus Foundation
- #AF-930351, #AF-939721 and #AF-968270/Swedish Alzheimer Foundation
- #RDAPB-201809-2016615/Alzheimer's Drug Discovery Foundation
- 101109451-ADEEPSLEEP/European Union's Marie Skłodowska-Curie Actions
- #201809-2016862/Alzheimer's Drug Discovery Foundation
- #WE.03-2019-02/Alzheimer Nederland
- #1R01AG068398-01/NH/NIH HHS/United States
- 101053962/European Union's Horizon Europe research and innovation programme
- AARG-22-920434/ALZ/Alzheimer's Association/United States
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

