Intensified conditioning containing decitabine versus standard myeloablative conditioning for adult patients with KMT2A-rearranged leukemia: a multicenter retrospective study
- PMID: 39736728
- PMCID: PMC11687046
- DOI: 10.1186/s12916-024-03830-0
Intensified conditioning containing decitabine versus standard myeloablative conditioning for adult patients with KMT2A-rearranged leukemia: a multicenter retrospective study
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is recommended for patients with KMT2A-rearranged (KMT2A-r) leukemia whereas relapse remains high. We aimed to determine whether intensified conditioning containing decitabine (Dec) could reduce relapse compared with standard myeloablative conditioning in adult patients with KMT2A-r leukemia.
Methods: We performed a multicenter retrospective study at seven institutions in China. Eligible patients were aged 14 years or older at transplantation, had a diagnosis of KMT2A-r leukemia, and underwent the first allo-HSCT. Standard myeloablative conditioning regimens (standard group) included BuCy (busulfan 3.2 mg/kg/day on days -7 to -4; cyclophosphamide 60 mg/kg/day on days -3 to -2) and TBI-Cy (total body irradiation 4.5 Gy/day on days -5 to -4; Cy 60 mg/kg/day on days -3 to -2). Intensified conditioning regimens containing Dec (intensified group) consisted of Dec-BuCy (Dec 20 mg/m2/day on days -14 to -10; the same dose of BuCy) and Dec-TBI-Cy (Dec 20 mg/m2/day on days -10 to -6; the same dose of TBI-Cy).
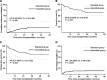
Results: Between April 2009 and December 2019, 218 patients were included in this study, of whom 105 were in the intensified group and 113 were in the standard group. The 3-year cumulative incidence of relapse was 17.6% and 34.5%, overall survival was 71.3% and 61.0%, disease-free survival was 70.1% and 56.0%, and non-relapse mortality was 12.3% and 9.5% in the intensified and standard groups, respectively (P = 0.001; P = 0.034; P = 0.005; P = 0.629). Subgroup analysis showed that the relapse rate of intensified conditioning was lower than that of standard conditioning in multiple subgroups, including different leukemia types, disease status at transplantation, high-risk cytogenetics and Bu-based regimens. There was no difference in regimen-related toxicity, engraftment, or graft-versus-host disease between the intensified and standard groups.
Conclusions: These results suggest that intensified conditioning containing Dec might be a better strategy than standard myeloablative conditioning for adult patients with KMT2A-r leukemia undergoing allo-HSCT.
Keywords: Allo-HSCT; Decitabine; Intensified conditioning; KMT2A-r leukemia.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the ethics committee review board at each participating center and was conducted in accordance with the principles of the Declaration of Helsinki. The reference number for the ethics committee was NFEC-2009–23. Written informed consent was obtained from all the patients. Consent for publication: All of the authors agreed to submit and publish the final manuscript. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
[Efficacy and Safety of Allogeneic Hematopoietic Stem Cell Transplantation with Decitabine-containing Regimen in Myelodysplastic Syndromes and Myelodysplastic Syndromes Transformed Acute Myeloid Leukemia].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023 Apr;31(2):522-531. doi: 10.19746/j.cnki.issn.1009-2137.2023.02.031. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023. PMID: 37096529 Chinese.
-
Busulfan Once Versus Four Times Daily: Impact on Pharmacokinetics, Organ Toxicities and Survival After Allogeneic Hematopoietic Stem Cell Transplantation.Transplant Cell Ther. 2025 Aug;31(8):571-583. doi: 10.1016/j.jtct.2025.04.019. Epub 2025 May 13. Transplant Cell Ther. 2025. PMID: 40373977
-
Post-transplant cyclophosphamide and early mixed donor Chimerism in myeloid malignancies; a single-center experience.Transpl Immunol. 2023 Apr;77:101808. doi: 10.1016/j.trim.2023.101808. Epub 2023 Feb 24. Transpl Immunol. 2023. PMID: 36842566
-
Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta-analysis.Hematol Oncol Stem Cell Ther. 2011;4(1):17-29. doi: 10.5144/1658-3876.2011.17. Hematol Oncol Stem Cell Ther. 2011. PMID: 21460603
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2014 Apr 20;2014(4):CD010189. doi: 10.1002/14651858.CD010189.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Nov 7;11:CD010189. doi: 10.1002/14651858.CD010189.pub3. PMID: 24748537 Free PMC article. Updated.
Cited by
-
Decitabine combined with reduced-intensity conditioning for older patients with acute myeloid leukemia in composite complete remission undergoing allogeneic hematopoietic stem cell transplantation: a multicenter, single-arm, phase 2 trial.Lancet Reg Health West Pac. 2025 Aug 12;61:101664. doi: 10.1016/j.lanwpc.2025.101664. eCollection 2025 Aug. Lancet Reg Health West Pac. 2025. PMID: 40831735 Free PMC article.
References
-
- van Weelderen RE, Klein K, Harrison CJ, et al. Measurable Residual Disease and Fusion Partner Independently Predict Survival and Relapse Risk in Childhood KMT2A-Rearranged Acute Myeloid Leukemia: A Study by the International Berlin-Frankfurt-Münster Study Group. J Clin Oncol. 2023;41(16):2963–74. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82293634/National Natural Science Foundation of China
- 82170213/National Natural Science Foundation of China
- 2021YFC2500300-4/National Key Research and Development Program of China
- 2022YFC2502600-5/National Key Research and Development Program of China
- 2024A1515010794/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

