Enhanced fetal hemoglobin production via dual-beneficial mutation editing of the HBG promoter in hematopoietic stem and progenitor cells for β-hemoglobinopathies
- PMID: 39736768
- PMCID: PMC11687217
- DOI: 10.1186/s13287-024-04117-0
Enhanced fetal hemoglobin production via dual-beneficial mutation editing of the HBG promoter in hematopoietic stem and progenitor cells for β-hemoglobinopathies
Abstract
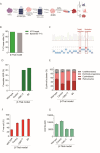
Background: Sickle cell disease (SCD) and β-thalassemia patients with elevated gamma globin (HBG1/G2) levels exhibit mild or no symptoms. To recapitulate this natural phenomenon, the most coveted gene therapy approach is to edit the regulatory sequences of HBG1/G2 to reactivate them. By editing more than one regulatory sequence in the HBG promoter, the production of fetal hemoglobin (HbF) can be significantly increased. However, achieving this goal requires precise nucleotide conversions in hematopoietic stem and progenitor cells (HSPCs) at therapeutic efficiency, which remains a challenge.
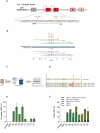
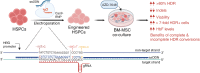
Methods: We employed Cas9 RNP-ssODN-mediated homology-directed repair (HDR) gene editing to mimic two naturally occurring HBG promoter point mutations; -175T > C, associated with high HbF levels, and -158 C > T, a common polymorphism in the Indian population that induces HbF under erythropoietic stress, in HSPCs.
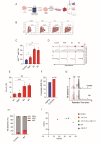
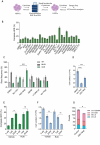
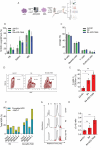
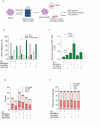
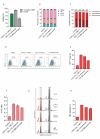
Results: Asymmetric, nontarget ssODN induced high rates of complete HDR conversions, with at least 15% of HSPCs exhibiting both the -175T > C and -158 C > T mutations. Optimized conditions and treatment with the small molecule AZD-7648 increased this rate, with up to 57% of long-term engrafting human HSPCs in NBSGW mice containing at least one beneficial mutation. Functionally, in vivo erythroblasts exhibited high levels of HbF, which was sufficient to reverse the cellular phenotype of β-thalassemia. Further support through bone marrow MSC co-culture boosted complete HDR conversion rates to exceed 80%, with minimal InDels, improved cell viability, and induced fetal hemoglobin levels similar to those of Cas9 RNP-mediated indels at BCL11A enhancer and HBG promoter.
Conclusions: Cas9 RNP-ssODN-based nucleotide conversion at the HBG promoter offers a promising gene therapy approach to ameliorate the phenotypes of β-thalassemia and SCD. The developed approach can simplify and broaden applications that require the cointroduction of multiple nucleotide modifications in HSPCs.
Keywords: Gene therapy; Hematopoietic stem cells; Homology-directed gene editing; Single-stranded oligonucleotides; β-hemoglobinopathies.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study titled “ In vivo efficacy and safety studies of CSCR-ST04, the gene edited autologous hematopoietic stem cells for the gene therapy of -hemoglobinopathies” was approved by the IRB of the Christian Medical College Vellore, with the approval number “IRB: 11807 (other) dated: 30.01.2019” and, the animal experiments were approved by the IAEC (approval number: 01/2019). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

