Platelet signaling in immune landscape: comprehensive mechanism and clinical therapy
- PMID: 39736771
- PMCID: PMC11686937
- DOI: 10.1186/s40364-024-00700-y
Platelet signaling in immune landscape: comprehensive mechanism and clinical therapy
Abstract
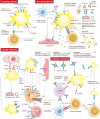
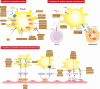
Platelets are essential for blood clotting and maintaining normal hemostasis. In pathological conditions, platelets are increasingly recognized as crucial regulatory factors in various immune-mediated inflammatory diseases. Resting platelets are induced by various factors such as immune complexes through Fc receptors, platelet-targeting autoantibodies and other platelet-activating stimuli. Platelet activation in immunological processes involves the release of immune activation stimuli, antigen presentation and interaction with immune cells. Platelets participate in both the innate immune system (neutrophils, monocytes/macrophages, dendritic cells (DCs) and Natural Killer (NK) cells and the adaptive immune system (T and B cells). Clinical therapeutic strategies include targeting platelet activation, platelet-immune cell interaction and platelet-endothelial cell interaction, which display positive development prospects. Understanding the mechanisms of platelets in immunity is important, and developing targeted modulations of these mechanisms will pave the way for promising therapeutic strategies.
Keywords: Clinical strategies; Immunity; Inflammation; Platelet.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no competing interests. Patient consent for publication: Not applicable. Ethics statement: Not applicable.
Figures

Similar articles
-
Innate immune receptors in platelets and platelet-leukocyte interactions.J Leukoc Biol. 2020 Oct;108(4):1157-1182. doi: 10.1002/JLB.4MR0620-701R. Epub 2020 Aug 10. J Leukoc Biol. 2020. PMID: 32779243 Review.
-
Platelet, a key regulator of innate and adaptive immunity.Front Med (Lausanne). 2023 Mar 10;10:1074878. doi: 10.3389/fmed.2023.1074878. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36968817 Free PMC article. Review.
-
Platelets and inter-cellular communication in immune responses: Dialogue with both professional and non-professional immune cells.Adv Protein Chem Struct Biol. 2024;140:347-379. doi: 10.1016/bs.apcsb.2023.12.010. Epub 2024 Apr 4. Adv Protein Chem Struct Biol. 2024. PMID: 38762274 Review.
-
Platelets in inflammation and immune modulations: functions beyond hemostasis.Arch Immunol Ther Exp (Warsz). 2012 Dec;60(6):443-51. doi: 10.1007/s00005-012-0193-y. Epub 2012 Sep 1. Arch Immunol Ther Exp (Warsz). 2012. PMID: 22940877 Review.
-
Beyond Hemostasis: Platelet Innate Immune Interactions and Thromboinflammation.Int J Mol Sci. 2022 Mar 31;23(7):3868. doi: 10.3390/ijms23073868. Int J Mol Sci. 2022. PMID: 35409226 Free PMC article. Review.
Cited by
-
Real-world experience of hetrombopag in immune thrombocytopenia treatment: a retrospective cohort study.Eur J Med Res. 2025 Jul 5;30(1):574. doi: 10.1186/s40001-025-02850-7. Eur J Med Res. 2025. PMID: 40615922 Free PMC article.
-
Platelets in infection: intrinsic roles and functional outcomes.Front Immunol. 2025 Jul 7;16:1616783. doi: 10.3389/fimmu.2025.1616783. eCollection 2025. Front Immunol. 2025. PMID: 40692784 Free PMC article. Review.
-
Platelets in Hepatocellular Carcinoma-From Pathogenesis to Targeted Therapy.Cancers (Basel). 2025 Jul 18;17(14):2391. doi: 10.3390/cancers17142391. Cancers (Basel). 2025. PMID: 40723273 Free PMC article. Review.
-
The gut-immune axis in primary immune thrombocytopenia (ITP): a paradigm shifts in treatment approaches.Front Immunol. 2025 Jun 12;16:1595977. doi: 10.3389/fimmu.2025.1595977. eCollection 2025. Front Immunol. 2025. PMID: 40574831 Free PMC article. Review.
-
Immunothrombosis in Sepsis: Cellular Crosstalk, Molecular Triggers, and Therapeutic Opportunities-A Review.Int J Mol Sci. 2025 Jun 25;26(13):6114. doi: 10.3390/ijms26136114. Int J Mol Sci. 2025. PMID: 40649892 Free PMC article. Review.
References
-
- van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16. 10.1038/s41569-018-0110-0. 166 – 79. - PubMed
-
- Nicolai L, Gaertner F, Massberg S. Platelets in host defense: experimental and clinical insights. Trends Immunol. 2019;40. 10.1016/j.it.2019.08.004. 922 – 38. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

