Dual inhibition of LAG-3 and PD-1 with IBI110 and sintilimab in advanced solid tumors: the first-in-human phase Ia/Ib study
- PMID: 39736787
- PMCID: PMC11687176
- DOI: 10.1186/s13045-024-01651-5
Dual inhibition of LAG-3 and PD-1 with IBI110 and sintilimab in advanced solid tumors: the first-in-human phase Ia/Ib study
Abstract
Background: Co-inhibition of immune checkpoints lymphocyte-activation gene 3 (LAG-3) and PD-1 is believed to enhance cancer immunotherapy through synergistic effects. Herein, we evaluate the safety and efficacy of IBI110 (anti-LAG-3 antibody) with sintilimab (an anti-PD-1 antibody) in Chinese patients with advanced solid tumors.
Methods: In this open-label phase I study, phase Ia dose escalation of IBI110 monotherapy and phase Ib combination dose escalation of IBI110 plus sintilimab were conducted in patients with advanced solid tumors. Additionally, phase Ib combination dose expansion of IBI110 plus sintilimab and chemotherapy was conducted in previously untreated, advanced squamous non-small cell lung cancer (sqNSCLC) and HER-2 negative gastric cancer (GC). In phase Ia dose escalation, patients received IBI110 monotherapy at 0.01/0.1/0.3/1/3/10/20 mg/kg Q3W. In phase Ib dose escalation, patients received IBI110 at 0.3/0.7/1.5/3/5/8/10 mg/kg Q3W plus sintilimab 200 mg Q3W. In phase Ib combination dose expansion, patients received IBI110 at recommended phase 2 dose (RP2D) plus sintilimab 200 mg Q3W and chemotherapy. The primary endpoints were safety, tolerability and efficacy including objective response rate (ORR), disease control rate (DCR), duration of response (DoR), progression-free survival (PFS) assessed by RECIST v1.1 and overall survival (OS). The secondary endpoints included pharmacokinetics, pharmacodynamics and immunogenicity.
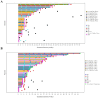
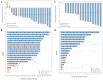
Results: In phase Ia dose escalation (n = 28), treatment-related adverse events (TRAEs) occurred in 67.9% patients and grade ≥ 3 TRAEs occurred in 21.4% patients. In phase Ib combination dose escalation (n = 45), TRAEs occurred in 75.6% patients and grade ≥ 3 TRAEs occurred in 22.2% patients. No dose-limiting toxicity (DLT) was observed. The most common TRAE was anemia (17.9%, including 3.6% ≥ G3) in phase Ia dose escalation of IBI110 monotherapy (n = 28), aspartate aminotransferase increased (28.9%, all G1-G2) in phase Ib dose escalation of IBI110 plus sintilimab (n = 45), anemia (70.0%, all G1-G2) in phase Ib dose expansion in sqNSCLC (n = 20), and neutrophil count decreased (64.7%, including 17.6%≥ G3) in phase Ib dose expansion in GC (n = 17). The RP2D of IBI110 was determined at 200 mg (3 mg/kg) Q3W. ORR in phase Ia/Ib dose escalation was 3.6% with IBI110 monotherapy and 14% with IBI110 plus sintilimab. In phase Ib combination dose expansion of IBI110 plus sintilimab and chemotherapy, unconfirmed and confirmed ORR in sqNSCLC (n = 20) was 80.0% (95% CI, 56.3-94.3) and 75.0% (95% CI, 50.9-91.3), respectively and in GC (n = 17) was 88.2% (95% CI, 63.6-98.5) and 70.6% (95% CI, 44.0-89.7), respectively.
Conclusions: IBI110 monotherapy and in combination with sintilimab were well-tolerated in Chinese patients with advanced solid tumors. Encouraging efficacy of IBI110 in combination with sintilimab and chemotherapies was observed in sqNSCLC and GC.
Trial registration: ClinicalTrials.gov Identifier: NCT04085185.
Keywords: Gastric cancer; LAG-3; Monoclonal antibody; Non-small cell lung cancer; PD-1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study protocol was approved by the institutional review board and ethics committee of all participating sites. This study was registered at clinicaltrials.gov (NCT04085185) and conducted following Good Clinical Practice Guidelines, the Declaration of Helsinki, and relevant local regulatory policy. Written informed consents were required before patient enrollment. Competing interests: The authors declare no competing interests.
Figures
References
-
- Borgeaud M, Sandoval J, Obeid M, Banna G, Michielin O, Addeo A et al. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treatment Reviews [Internet]. 2023 [cited 2023 Dec 5];120. https://www.cancertreatmentreviews.com/article/S0305-7372(23)00107-X/ful... - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

