Risdiplam utilization, adherence, and associated health care costs for patients with spinal muscular atrophy: a United States retrospective claims database analysis
- PMID: 39736792
- PMCID: PMC11684252
- DOI: 10.1186/s13023-024-03399-0
Risdiplam utilization, adherence, and associated health care costs for patients with spinal muscular atrophy: a United States retrospective claims database analysis
Abstract
Background: Spinal muscular atrophy (SMA) is a genetic neuromuscular disease associated with progressive loss of motor function. Risdiplam, a daily oral therapy, was approved in the United States for the treatment of SMA. Risdiplam's effectiveness depends on patient adherence to the treatment regimen. This retrospective claims database analysis assessed real-world treatment adherence and persistence, and all-cause health care costs by adherence status, for patients with SMA receiving risdiplam. Outcomes were summarized by SMA types (1-4) and age groups (0-2, 3-5, 6-17, and ≥ 18 years).
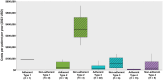
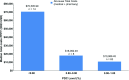
Results: 86 patients with ≥ 1 SMA diagnosis, risdiplam treatment, and ≥ 6 months of continuous enrollment after the index date (SMA diagnosis) were identified in the IQVIA PharMetrics® Plus database (01/01/2020-06/30/2022). One patient had SMA type 1 (a 1-year-old boy), 18 had type 2 (mean ± SD age: 7.9 ± 5.7 years; 61% female), 47 had type 3 (17.3 ± 10.2 years; 55% female), and 20 had type 4 (38.2 ± 11.6 years; 55% female). The mean proportion of days covered (PDC) with risdiplam was 0.89 overall, ranging from 0.88 for SMA type 4 to 0.97 for type 1. The majority (83.7%) of patients were adherent to risdiplam (PDC ≥0.80), ranging from 75.0% for type 4 to 100% for type 1. Adherence ranged from 76.5% among 6-12-year-olds to 100% among 0-2-year-olds. Compared with adherent patients, nonadherent patients had higher median total health care costs by $335,049 for type 2, $41,204 for type 3, and $12,223 for type 4. Among adherent patients, patients with PDC between 0.90 and 1.00 had lower costs compared with patients with PDC between 0.80 and 0.90.
Conclusions: Nonadherence to risdiplam was observed in the first year of treatment, especially for patients with SMA type 4 and patients aged 6-12 years. Nonadherence was associated with higher all-cause health care costs, with the most pronounced cost difference for SMA type 2. For adherent patients, those who were highly adherent incurred lower health care costs. These findings underscore the importance of treatment adherence and persistence for patients with SMA receiving risdiplam, particularly for younger children and those with greater disease severity.
Keywords: Adherence; Claims database analysis; Health care costs; Health care utilization; Persistence; Risdiplam; Spinal muscular atrophy; United States.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study utilized data routinely collected by the payers of the health services, which was de-identified to ensure the protection of individual identities. Consent for publication: Not applicable. Competing interests: AP, WT, and OD are employees of Novartis Gene Therapies, Inc., and own stock/options. MYa, WS, RD, MYe, and NT are employees of Analysis Group, Inc., which has received consulting fees from Novartis Gene Therapies, Inc., for this study.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

