Taltirelin induces TH expression by regulating TRHR and RARα in medium spiny neurons
- PMID: 39736794
- PMCID: PMC11687129
- DOI: 10.1186/s12967-024-06020-x
Taltirelin induces TH expression by regulating TRHR and RARα in medium spiny neurons
Abstract
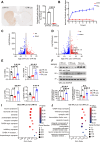
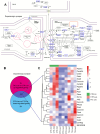
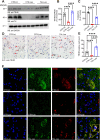
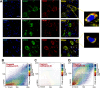
Taltirelin, an orally effective thyrotropin-releasing hormone analog, significantly improves motor impairments in rat models of Parkinson's disease (PD) and enhances dopamine release within the striatum. However, the underlying mechanism remains unclear. In this study, a variety of in vivo and in vitro methods, including transcriptomic analysis, were employed to elucidate the effects of Taltirelin on cellular composition and signaling pathways in the striatum of hemi-PD rats. We demonstrated that Taltirelin upregulates the expression of TRHR on striatal GABAergic neurons, which is accompanied by activation of the TRHR-MAPK-RARα-DRD2 pathway. Consequently, Taltirelin induces medium spiny neurons in the striatum to express TH. This discovery provides valuable insights into the potential application of Taltirelin in neurological disorders and offers new directions for drug development.
Keywords: Dopamine receptor D2; Taltirelin; Thyrotropin-releasing hormone receptor; Tyrosine hydroxylase.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing financial interests.
Figures




Similar articles
-
TRH Analog, Taltirelin Improves Motor Function of Hemi-PD Rats Without Inducing Dyskinesia via Sustained Dopamine Stimulating Effect.Front Cell Neurosci. 2018 Nov 13;12:417. doi: 10.3389/fncel.2018.00417. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30555300 Free PMC article.
-
Lack of behavioral tolerance by repeated treatment with taltirelin hydrate, a thyrotropin-releasing hormone analog, in rats.Pharmacol Biochem Behav. 2005 Dec;82(4):646-51. doi: 10.1016/j.pbb.2005.11.004. Epub 2005 Dec 20. Pharmacol Biochem Behav. 2005. PMID: 16368129
-
Inhibition of microRNA-200a Upregulates the Expression of Striatal Dopamine Receptor D2 to Repress Apoptosis of Striatum via the cAMP/PKA Signaling Pathway in Rats with Parkinson's Disease.Cell Physiol Biochem. 2018;51(4):1600-1615. doi: 10.1159/000495649. Epub 2018 Nov 29. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2021;55(5):675. doi: 10.33594/000000465. PMID: 30497067 Retracted.
-
D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
-
Progress in pursuit of therapeutic A2A antagonists: the adenosine A2A receptor selective antagonist KW6002: research and development toward a novel nondopaminergic therapy for Parkinson's disease.Neurology. 2003 Dec 9;61(11 Suppl 6):S97-100. doi: 10.1212/01.wnl.0000095219.22086.31. Neurology. 2003. PMID: 14663020 Review.
References
-
- Shimizu T, Tsutsumi R, Shimizu K, Tominaga N, Nagai M, Ugawa Y, et al. Differential effects of thyrotropin releasing hormone (TRH) on motor execution and motor adaptation process in patients with spinocerebellar degeneration. J Neurol Sci. 2020;415: 116927. - PubMed
-
- Kelly JA, Boyle NT, Cole N, Slator GR, Colivicchi MA, Stefanini C, et al. First-in-class thyrotropin-releasing hormone (TRH)-based compound binds to a pharmacologically distinct TRH receptor subtype in human brain and is effective in neurodegenerative models. Neuropharmacology. 2015;89:193–203. - PubMed
-
- Duval F, Mokrani M-C, Erb A, Danila V, Lopera FG, Foucher JR, et al. Thyroid axis activity and dopamine function in depression. Psychoneuroendocrinology. 2021;128: 105219. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

