Insights into the biology and insecticide susceptibility of the secondary malaria vector Anopheles parensis in an area with long-term use of insecticide-treated nets in northwestern Tanzania
- PMID: 39736795
- PMCID: PMC11687015
- DOI: 10.1186/s13071-024-06634-6
Insights into the biology and insecticide susceptibility of the secondary malaria vector Anopheles parensis in an area with long-term use of insecticide-treated nets in northwestern Tanzania
Abstract
Background: The Anopheles funestus group includes at least 11 sibling species, with Anopheles funestus Giles being the most studied and significant malaria vector. Other species, like Anopheles parensis, are understudied despite their potential role in transmission. This article provides insights into the biology and insecticide susceptibility of An. parensis, with observations of its densities in northwestern Tanzania.
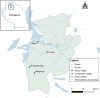
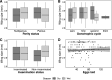
Methods: Mosquitoes were collected in three villages in Misungwi district, northwestern Tanzania, using CDC light traps and battery-powered aspirators indoors and human-baited double net traps outdoors. Female Anopheles adults were morphologically sorted and identified by PCR, and a subset was tested by ELISA for vertebrate blood meal sources and Plasmodium sporozoite infections. Insecticide susceptibility was assessed using the WHO protocol (2nd edition, 2018). Unfed females were dissected to assess parity, gonotrophic status and insemination status, while blood-fed females were monitored for oviposition to estimate egg counts. The prevalence of An. parensis was generally < 24% across all sites, except in Ngaya village, where it unexpectedly constituted 84% of PCR-amplified An. funestus sensu lato. This species was present in both indoor and outdoor collections, yet the females exclusively fed on non-human vertebrates, with no human blood meals detected. Parity rates were approximately 49% for resting and 46% for host-seeking females, with slightly higher percentages of both parous and inseminated females in the dry season compared to the wet season. Most parous females had oviposited once or twice, with those in the dry season ovipositing significantly more eggs. The average wing length of female An. parensis was 2.93 mm, and there was no significant impact of body size on parity, fecundity or insemination. The An. parensis mosquitoes were fully susceptible to pyrethroids, carbamates, organophosphates and organochlorides.
Results: The prevalence of An. parensis was generally < 24% across all sites, except in Ngaya village, where it unexpectedly constituted 84% of PCR-amplified An. funestus sensu lato. This species was present in both indoor and outdoor collections, yet the females exclusively fed on non-human vertebrates, with no human blood meals detected. Parity rates were approximately 49% for resting and 46% for host-seeking females, with slightly higher percentages of both parous and inseminated females in the dry season compared to the wet season. Most parous females had oviposited once or twice, with those in the dry season ovipositing significantly more eggs. The average wing length of female An. parensis was 2.93 mm, and there was no significant impact of body size on parity, fecundity or insemination. The An. parensis mosquitoes were fully susceptible to pyrethroids, carbamates, organophosphates and organochlorides..
Conclusion: This study offers insights into the behaviours and insecticide susceptibility of An. parensis. Primarily feeding on non-human hosts, An. parensis is less significant in malaria transmission than more anthropophilic vectors. Unlike the pyrethroid-resistant An. funestus sensu stricto, An. parensis remains fully susceptible to public health insecticides despite the use of insecticidal bed nets. These findings provide a foundation for future research and may inform control strategies targeting residual malaria transmission involving An. parensis.
Keywords: Anopheles parensis; Plasmodium spp; Malaria; Tanzania.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approvals for this project were obtained from Ifakara Health Institute’s Institutional Review Board (Protocol ID: IHI/IRB/No: 19 – 2017 and IHI/IRB/EXT/No: 33—2022) and the Medical Research Coordinating Committee (MRCC) at the National Institute for Medical Research in Tanzania (Protocol ID: NIMR/HQ/R.8a/Vol.IX/3494). Written consents were sought from all participants of this study after they had understood the purpose and procedure of the discussions. Consent for publication: Permission to publish this study was obtained from National Institute for Medical Research in Tanzania (BD.242/437/01C/78). Competing interests: The authors declare no competing interests.
Figures

References
-
- Charlwood JD. The ecology of malaria vectors. Boca Raton: CRC Press; 2019. 10.1201/9780429284748
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

