Bacterial synergies amplify nitrogenase activity in diverse systems
- PMID: 39736847
- PMCID: PMC11684072
- DOI: 10.1093/ismeco/ycae158
Bacterial synergies amplify nitrogenase activity in diverse systems
Abstract
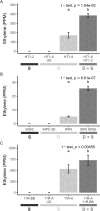
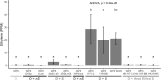
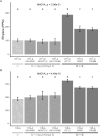
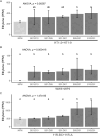
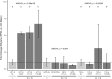
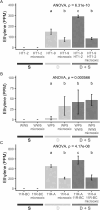
Endophytes are microbes living within plant tissue, with some having the capacity to fix atmospheric nitrogen in both a free-living state and within their plant host. They are part of a diverse microbial community whose interactions sometimes result in a more productive symbiosis with the host plant. Here, we report the co-isolation of diazotrophic endophytes with synergistic partners sourced from two separate nutrient-limited sites. In the presence of these synergistic strains, the nitrogen-fixing activity of the diazotroph is amplified. One such partnership was co-isolated from extracts of plants from a nutrient-limited Hawaiian lava field and another from the roots of Populus trees on a nutrient-limited gravel bar in the Pacific Northwest. The synergistic strains were capable of increasing the nitrogenase activity of different diazotrophic species from other environments, perhaps indicating that these endophytic microbial interactions are common to environments where nutrients are particularly limited. Multiple overlapping mechanisms seem to be involved in this interaction. Though synergistic strains are likely capable of protecting nitrogenase from oxygen, another mechanism seems evident in both environments. The synergies do not depend exclusively on physical contact, indicating a secreted compound may be involved. This work offers insights into beneficial microbial interactions, providing potential avenues for optimizing inocula for use in agriculture.
Keywords: endophytes; microbial interactions; nitrogen fixation; populus; symbiosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures


References
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

