Deciphering the roles of non-coding RNAs in liposarcoma development: Challenges and opportunities for translational therapeutic advances
- PMID: 39736850
- PMCID: PMC11683247
- DOI: 10.1016/j.ncrna.2024.11.005
Deciphering the roles of non-coding RNAs in liposarcoma development: Challenges and opportunities for translational therapeutic advances
Abstract
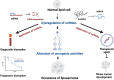
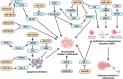
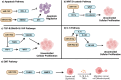
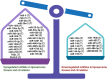
Liposarcoma is one of the most prevalent forms of soft tissue sarcoma, and its prognosis is highly dependent on its molecular subtypes. Non-coding RNAs (ncRNAs) like microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) can bind various cellular targets to regulate carcinogenesis. By affecting the expressions and activities of their downstream targets post-transcriptionally, dysregulations of miRNAs can alter different oncogenic signalling pathways, mediating liposarcoma progression. On the contrary, lncRNAs can sponge miRNAs to spare their downstream targets from translational repression, indirectly affecting miRNA-regulated oncogenic activities. In the past 15 years, multiple fundamental and clinical research has shown that different ncRNAs play essential roles in modulating liposarcoma development. Yet, there is a lack of an effective review report that could summarize the findings from various studies. To narrow this literature gap, this review article aimed to compare the findings from different studies on the tumour-regulatory roles of ncRNAs in liposarcoma and to understand how ncRNAs control liposarcoma progression mechanistically. Additionally, the reported findings were critically reviewed to evaluate the translational potentials of various ncRNAs in clinical applications, including employing these ncRNAs as diagnostic and prognostic biomarkers or as therapeutic targets in the management of liposarcoma. Overall, over 15 ncRNAs were reported to play essential roles in modulating different cellular pathways, including apoptosis, WNT/β-catenin, TGF-β/SMAD4, EMT, interleukin, and YAP-associated pathways to influence liposarcoma development. 28 ncRNAs were reported to be upregulated in liposarcoma tissues or circulation, whereas 11 were downregulated, making them potential candidates as liposarcoma diagnostic biomarkers. Among these ncRNAs, measuring the tissues or circulating levels of miR-155 and miR-195 was reported to help detect liposarcoma, differentiate liposarcoma subtypes, and predict the survival and treatment response of liposarcoma patients. Overall, except for a few ncRNAs like miR-155 and miR-195, current evidence to support the use of discussed ncRNAs as biomarkers and therapeutic targets in managing liposarcoma is mainly based on a single-center study with relatively small sample sizes or cell-based studies. Hence, more large-scale multi-center studies should be conducted to further confirm the sensitivity, specificity, and safety of ncRNAs as biomarkers and therapeutic targets. Instead of furthering investigation to confirm the translational values of all the discussed ncRNAs, which can be time- and cost-consuming, it would be more practical to focus on a few ncRNAs, including miR-155 and miR-195, to evaluate if they are sensitive and safe to be used as liposarcoma biomarkers and therapeutic agents or targets.
Keywords: Diagnosis; Liposarcoma; Prognosis; lncRNAs; miRNAs; ncRNAs.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Extracellular Vesicles as Delivery Vehicles for Non-Coding RNAs: Potential Biomarkers for Chronic Liver Diseases.Biomolecules. 2024 Feb 26;14(3):277. doi: 10.3390/biom14030277. Biomolecules. 2024. PMID: 38540698 Free PMC article. Review.
-
The emerging role of non-coding RNAs in the Wnt/β-catenin signaling pathway in Prostate Cancer.Pathol Res Pract. 2024 Feb;254:155134. doi: 10.1016/j.prp.2024.155134. Epub 2024 Jan 14. Pathol Res Pract. 2024. PMID: 38277746 Review.
-
Molecular functions of microRNAs in colorectal cancer: recent roles in proliferation, angiogenesis, apoptosis, and chemoresistance.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5617-5630. doi: 10.1007/s00210-024-03076-w. Epub 2024 Apr 15. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38619588 Review.
-
microRNA 21 and long non-coding RNAs interplays underlie cancer pathophysiology: A narrative review.Noncoding RNA Res. 2024 Mar 31;9(3):831-852. doi: 10.1016/j.ncrna.2024.03.013. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38586315 Free PMC article. Review.
-
Clinical relevance of circulating non-coding RNAs in metabolic diseases: Emphasis on obesity, diabetes, cardiovascular diseases and metabolic syndrome.Genes Dis. 2022 Jun 3;10(6):2393-2413. doi: 10.1016/j.gendis.2022.05.022. eCollection 2023 Nov. Genes Dis. 2022. PMID: 37554181 Free PMC article. Review.
References
-
- Tie T.L., Duski S. Outcome of patients with liposarcoma: a retrospective review over 12 Years in a single center. Clinical Surgical Oncology. 2024;3 doi: 10.1016/j.cson.2024.100043. - DOI
-
- Ciongariu A.-M., Țăpoi D.-A., Dumitru A.-V., Bejenariu A., Marin A., Costache M. Pleomorphic liposarcoma unraveled: investigating histopathological and immunohistochemical markers for tailored diagnosis and therapeutic innovations. Medicina. 2024;60:950. doi: 10.3390/medicina60060950. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

