Everolimus in pituitary tumor: a review of preclinical and clinical evidence
- PMID: 39736867
- PMCID: PMC11682973
- DOI: 10.3389/fendo.2024.1456922
Everolimus in pituitary tumor: a review of preclinical and clinical evidence
Abstract
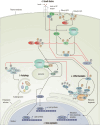
Although pituitary tumors (PTs) are mostly benign, some PTs are characterized by low surgical resection rates, high recurrence rates, and poor response to conventional treatments and profoundly affect patients' quality of life. Everolimus (EVE) is the only FDA-approved mTOR inhibitor, which can be used for oral treatment. It effectively inhibits tumor cell proliferation and angiogenesis. It has been administered for various neuroendocrine tumors of the digestive tract, lungs, and pancreas. EVE not only suppresses the growth and proliferation of APT cells but also enhances their sensitivity to radiotherapy and chemotherapy. This review introduces the role of the PI3K/AKT/mTOR pathway in the development of APTs, comprehensively explores the current status of preclinical and clinical research of EVE in APTs, and discusses the blood-brain barrier permeability and safety of EVE.
Keywords: Everolimus; PI3K/AKT/mTOR; blood-brain barrier; pituitary tumor; safety.
Copyright © 2024 Yao and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

